4. A container contains 32 g of O2 at a temperature TThe pressure
By A Mystery Man Writer
4. A container contains 32 g of O2 at a temperature TThe pressure of the gas is P. An identical containercontaining 4 g of H2 at a temperature 2T has apressure of(1) 8P(3) P(2) 4P(4) P18r cnstant
4- A container contains 32 g of O2 at a temperature TThe pressure of the gas is P- An identical containercontaining 4 g of H2 at a temperature 2T has apressure of-1- 8P-3- P-2- 4P-4- P18r-cnstant

Solved 1. A container with a volume of 6.0 L holds 10 g of

4. (3) Less back (4) Less A container contains 32 g of O, a temperature T. The pressure of the gas is P. An identical container containing 4 g of H, a
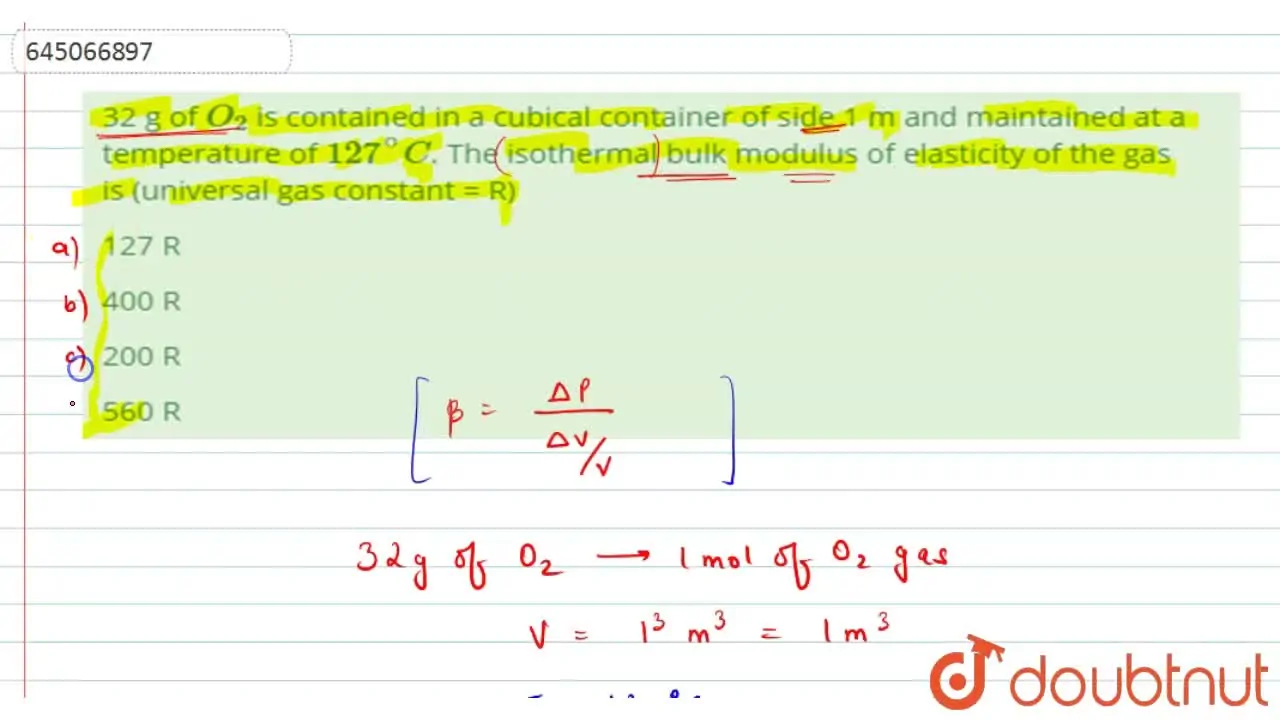
32 g of O(2) is contained in a cubical container of side 1 m and maint

A container contains 32 g of O, a temperature T. The pressure of the gas is P. An identical container containing 4 g of H, a temperature 2T has a pressure of (

A vessel contains 28 gm of N−2 and 32 gm of O2 temperature T = 1800 K and pressure 2 atm pressure it N2 dissociates 30 and O2 dissociates 50 temperature remains constant.

A vessel contains 32 g of O_2 at a temperature T. The pressure of the gas is p. An identical ves
The equilibrium constant (K) for the reaction,2SO2(g)+O2(g)2S03(g) at 1000 K is 3.5 atmWhat would be the partial pressure of oxygen gas,if the equilibrium is found to have equal moles ofSO2 and SO3?

Solved Question completion QUESTION 25 A container contains

Solved Problem 6. (Mass, concentration, and rms speed) and
- Chocolate Nestlé Chokito 32g Display com 30 unid.

- Smart Agulha de Lebel - 32G/4mm (Caixa com 100) - Smart GR - Smart GR

- Fralda Looping Looney Tunes Mega G 32 Unidades - Drogaria Venancio
- HALLS Rebuçados Mentol Sem Açúcar 32 g, CARAMELOS DUROS

- ViewSonic XG321UG 32 4K 144Hz Mini LED G-Sync Ultimate Gaming Monitor - ViewSonic Global

- Arctic Quest Womens Softshell Ski Pant - Water Resistant, Fleece

- Recycled Cotton Low-Rise Denim Shorts

- Patrick Star - Simple English Wikipedia, the free encyclopedia

- Why did Huggies do two versions of the Pull-ups New Leaf commercial with two different bears? : r/CommercialsIHate

- Wholesale Buttery Smooth Flirty Camouflage Plus Size Leggings
