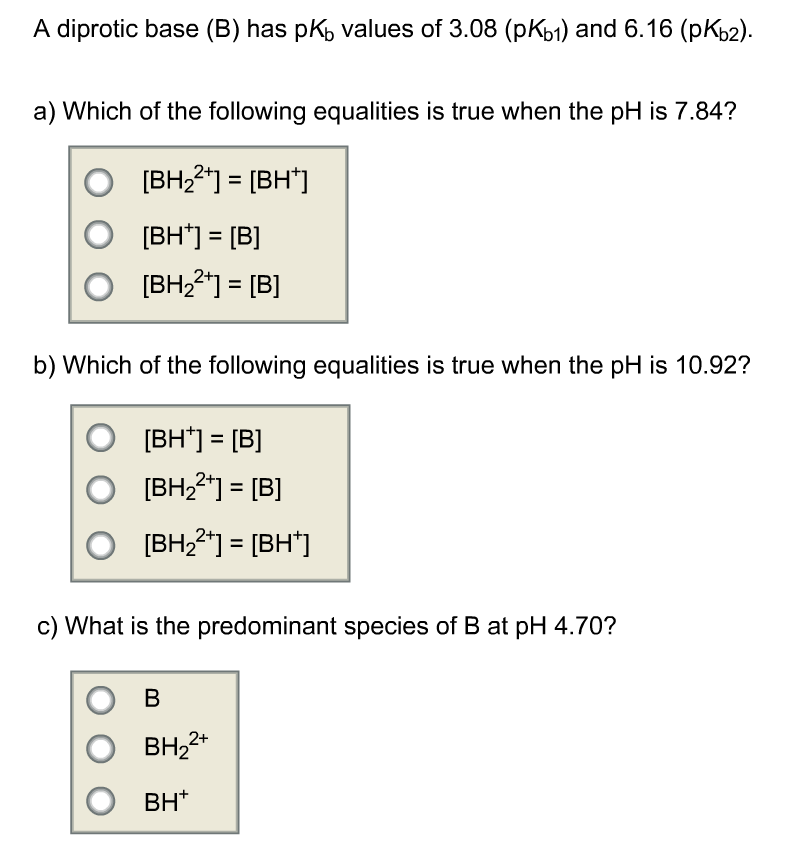
Solved A diprotic base (B) has pKb values of 3.08 (pKb1) and
By A Mystery Man Writer

SOLVED: A diprotic base B has pKa values of 3.54, 7.08, and 10.46. Which of the equalities is true when the pH is 6.92? [BH+] = [B] [BH] = [B] [BH+] = [

Equilibrium - Flip eBook Pages 51-100

PHARMACEUTICAL ANALYSIS I - ACID BASE TITRATIONS

Having trouble understanding diprotic acid-base equilibria. : r/chemhelp

PHARMACEUTICAL ANALYSIS I - ACID BASE TITRATIONS
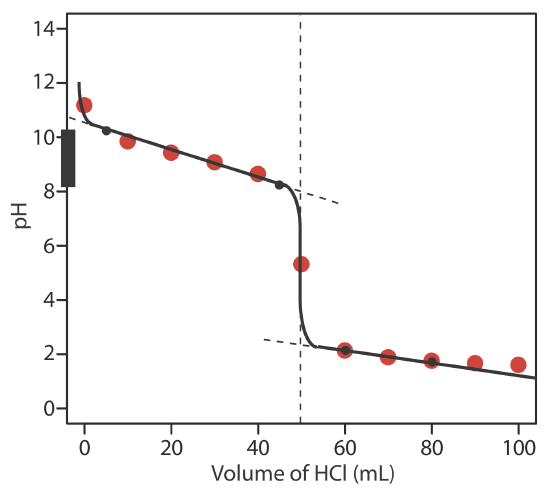
6.6: pH Calculations for Acid–Base Titrations - Chemistry LibreTexts
The pKb values for the dibasic base B are pKb1=2.10 and pKb2=7.54. Calculate the pH at each of the points

Equilibrium - Flip eBook Pages 51-100
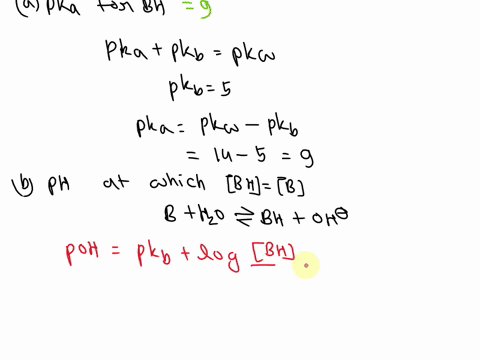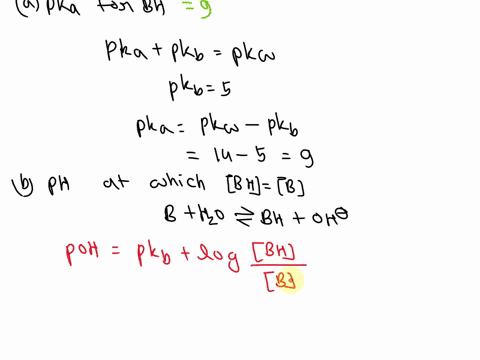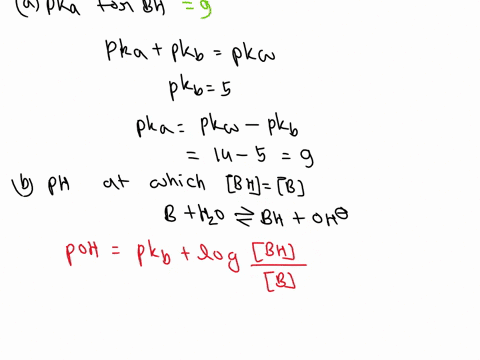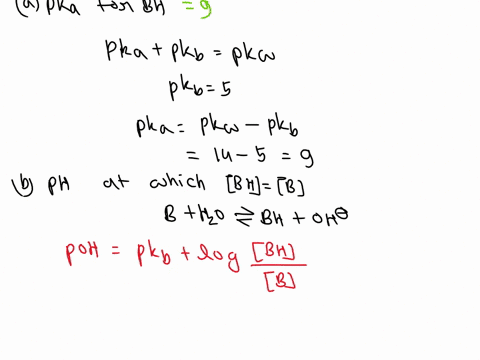
SOLVED: A diprotic base B has pKa values of 3.54, 7.08, and 10.46. Which of the equalities is true when the pH is 6.92? [BH+] = [B] [BH] = [B] [BH+] = [

Docdownloader Com 17-Petrucci10e-Csm PDF, PDF, Titration
- Bh beugel licht push up effect zwart naadloze cup Wacoal - Bodyfashion Born

- LOTE 13 - Mansão B. Mangabeiras - PROCESSO 0011242-61.2016- 27ª BH

- Initial Letter B H Logo Design Vector Template. Graphic Alphabet Symbol For Corporate Business Identity - MasterBundles

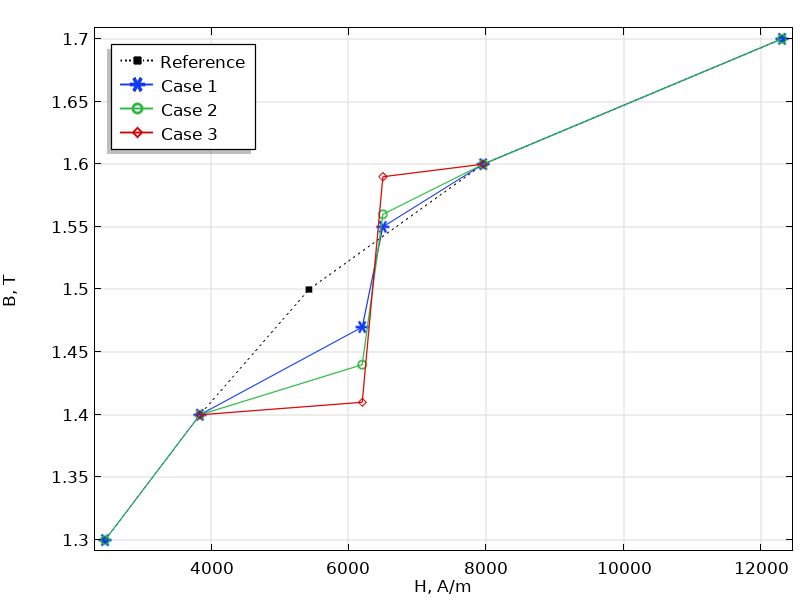
- How the B-H Curve Affects a Magnetic Analysis (and How to Improve
- BHB College Recruitment 2024 - Assistant Professor Vacancy

- TRIUMPH Essential Minimizer W Women Minimizer Non Padded Bra - Buy TRIUMPH Essential Minimizer W Women Minimizer Non Padded Bra Online at Best Prices in India

- Incoming Chicago Mayor Brandon Johnson Defends Teens after Weekend

- BAGS, POUCHES + PURSES - PLUSH HEART BAG MERI MERI

- My Posh Closet panosundaki Pin

- Push Up Bra For Women Bras None Underwire Brassiere
