Cyclohexane Chair Conformation Stability: Which One Is Lower Energy?

By A Mystery Man Writer
To determine chair conformation stability, add up the "A-Values" for each axial substituent. The lower that number is, the more stable the chair.
Cyclohexane conformation - Wikipedia

Cyclohexane Chair Conformation Stability: Which One Is Lower Energy?
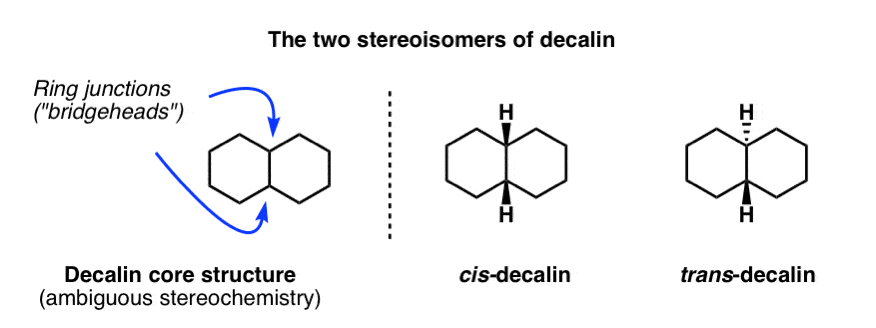
Fused Rings: Cis and Trans Decalin – Master Organic Chemistry
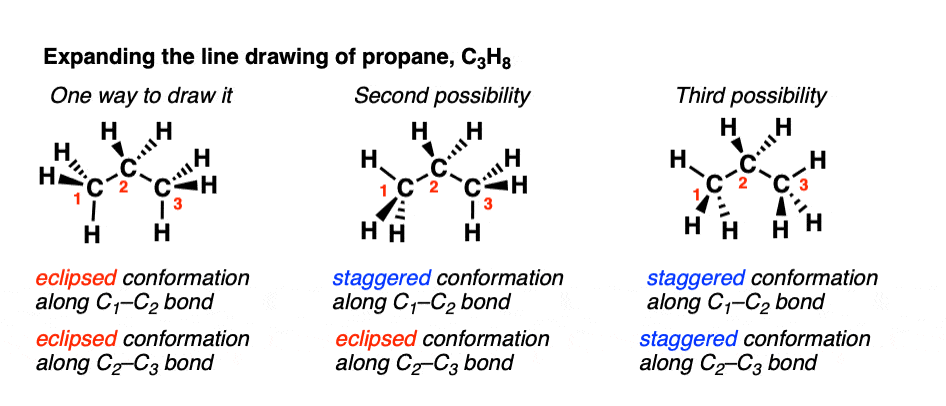
Conformational Isomers of Propane – Master Organic Chemistry

Draw the more stable chair conformation of the molecule, and estimate the amount of strain in trans-1-Chloro-3-methylcyclohexane.

Ring-Flip: Comparing the Stability of Chair Conformations with Practice Problems - Chemistry Steps

The diaxial conformation of cis-1,3-dimethylcyclohexane is approximately 23 rac{kJ}{mol} (5.4 rac{kcal}{mol}) less stable than the diequatorial conformation. Draw the two possible chair conformations, and suggest a reason for the large energy
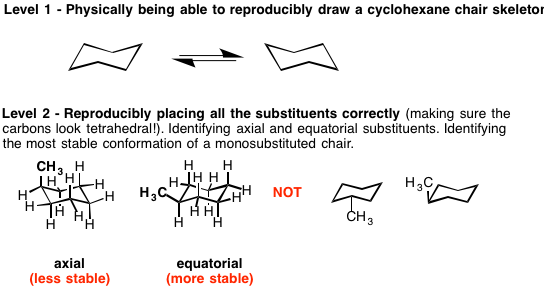
Levels of Mastery – Master Organic Chemistry
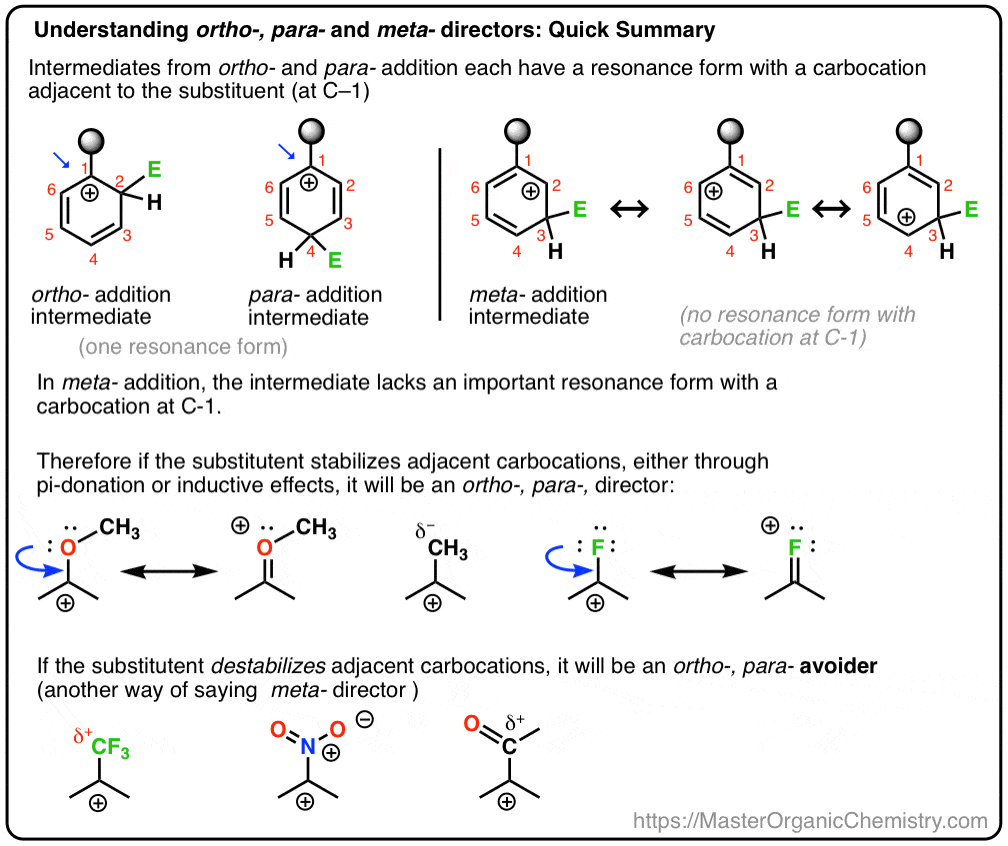
Understanding Ortho, Para, and Meta Directors - Master Organic Chemistry

Cyclohexane Chair Conformation Stability: Which One Is Lower Energy?

Ring-Flip: Comparing the Stability of Chair Conformations with Practice Problems - Chemistry Steps
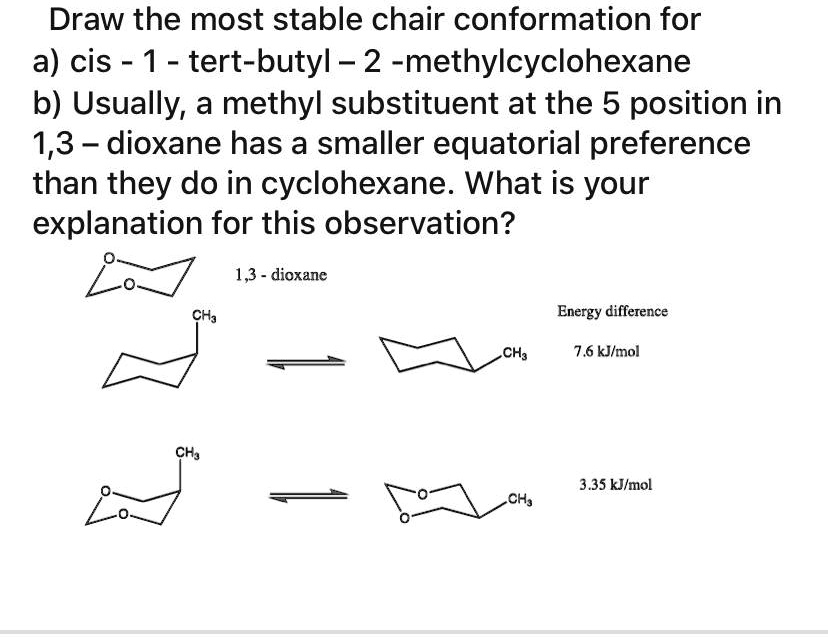
SOLVED: Draw the most stable chair conformation for a) cis-1-tert-butyl-2-methylcyclohexane. b) Usually, a methyl substituent at the 5 position in 1,3-dioxane has a smaller equatorial preference than they do in cyclohexane. What
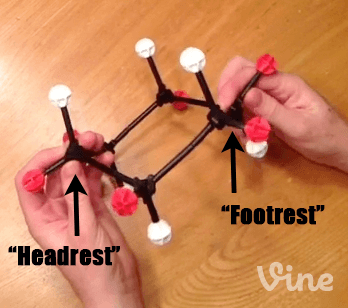
The Cyclohexane Chair Flip – Master Organic Chemistry








