Ideal–Universal Gas Law
By A Mystery Man Writer
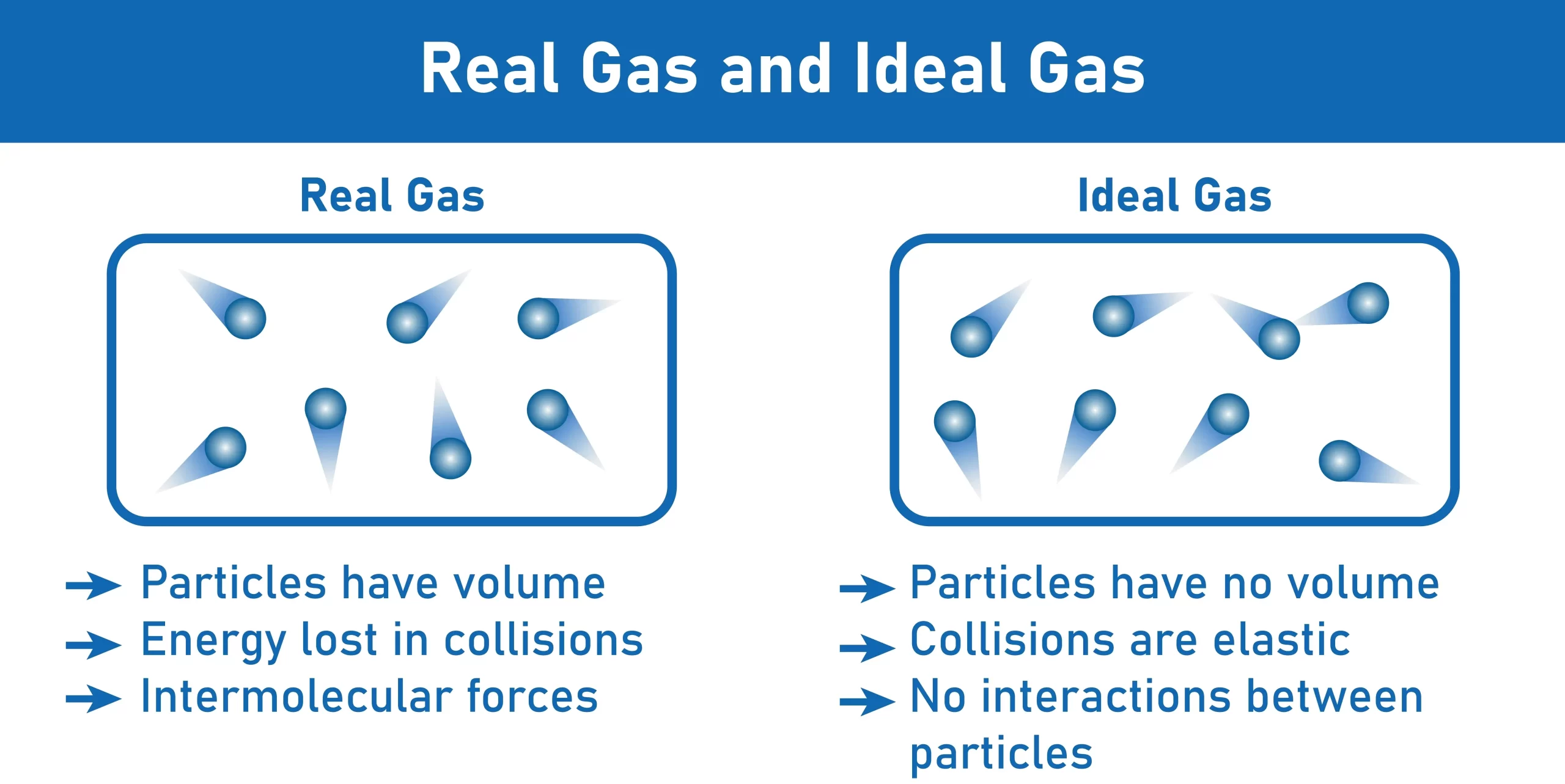
Definition: The Universal or Ideal Gas Law describes the relationship between all four properties (pressure, volume, number of moles, and temperature) as well as a gas constant called “R.” NOTE: The Ideal Gas Constant “R” has constant a value of 0.0821 L.atm/mol.K Relation: The relation between pressure (P) volume (V), number of moles (n) and…
Units of Ideal Gas Constant R and Density of an Ideal Gas - video Dailymotion

physical chemistry - What is the relation between universal gas constant R and amount of substance n? - Chemistry Stack Exchange

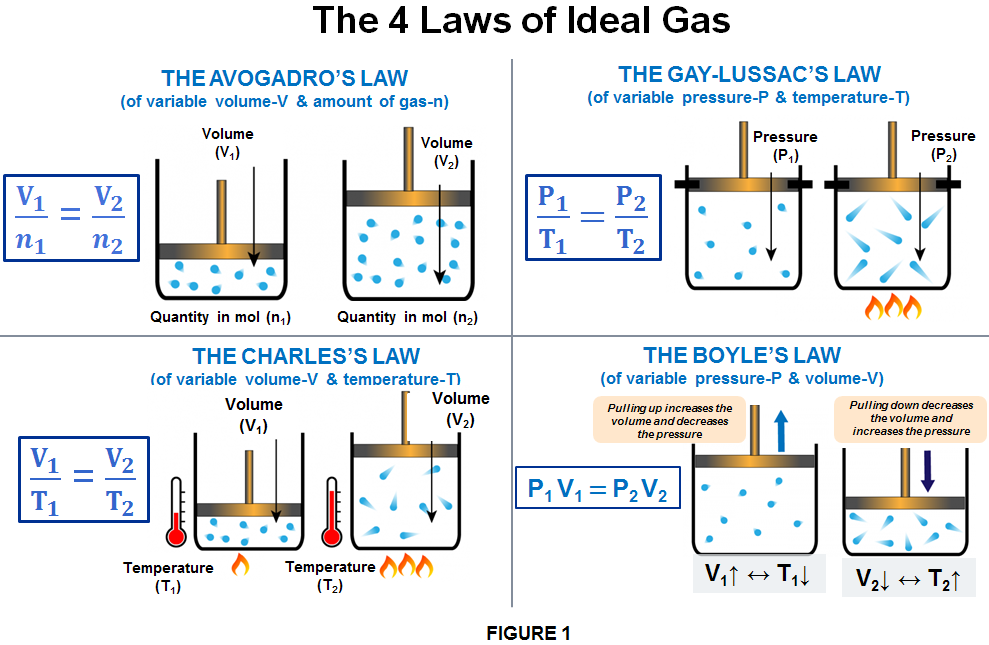
Avogadro's Law

Effective Nuclear Charge
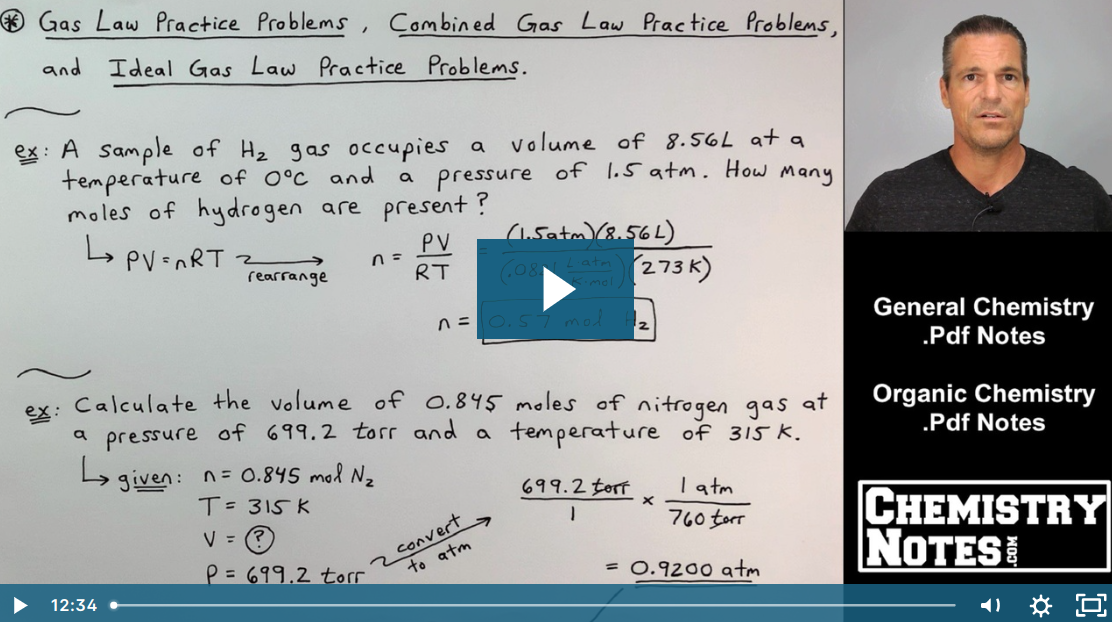
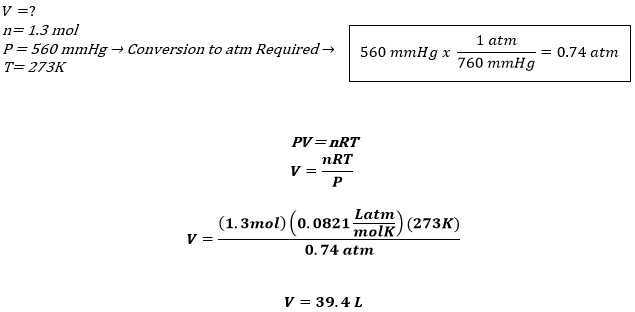
Gas Law Examples and Gas Law Calculations

MathType on X: The gas constant “R” is defined as the Avogadro constant “NA“ multiplied by the Boltzmann constant “k”. It is mostly known for appearing in the ideal gas law and
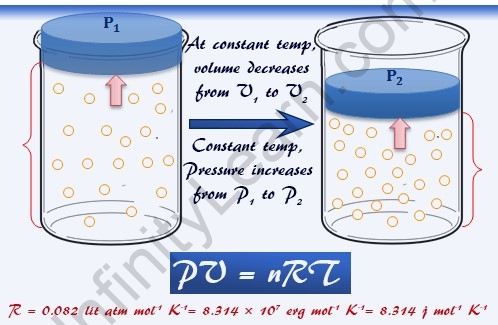
Dimensions Of Universal Gas Constant - Infinity Learn by Sri Chaitanya

Standard Temperature and Pressure

ANESTHESIA EQUIPMENT AND GAS LAW REVIEW - ppt download

Orbital Diagrams
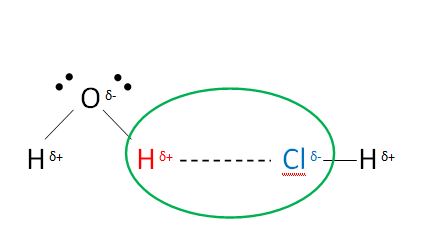
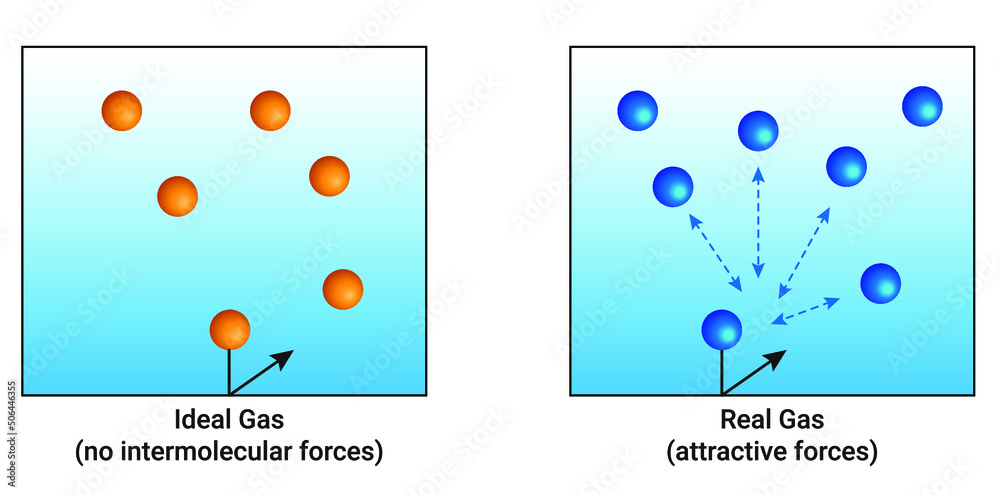
Intermolecular Forces of Attraction
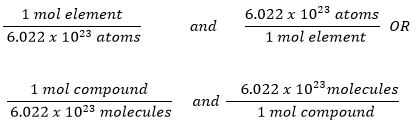
The Mole Concept: Molecules and Atoms

Regents Chemistry--Physical Setting Power Pack Revised Edition by Albert S. Tarendash (Ebook) - Read free for 30 days