Developing a Thermodynamical Method for Prediction of Activity Coefficient of TBP Dissolved in Kerosene

By A Mystery Man Writer
Results of the experimental measurements on the partial molar volume of kerosene used as a medium for dissolving TBP are utilized to determine the activity of TBP in the binary kerosene-TBP solution through the application of Gibbs-Duhem equation. The treatment is based on combination of the experimental data with the thermodynamic values available on the compressibility factor of pure kerosene at room temperature. It is shown that the activity of TBP in kerosene has a positive deviation from ideality with an activity coefficient derived as follows:1) at X TBP ≤ 0.01: γ TBP = 42.530, 2) at the 0.01 X TBP 0.2: 3) at the higher TBP concentrations 0.2 X TBP 0.97: and 4) at TBP Raoultian concentrations 0.97 ≤ X TBP:γ TBP = 1. These quantities can be utilized at temperature closed to 298 K.

Water adsorption in the organic phase for the D2EHPA-kerosene

Developing a Thermodynamical Method for Prediction of Activity Coefficient of TBP Dissolved in Kerosene

Thermodynamic & Kinetic Data for Sustainable Energy

The integral molar volume of TBP-kerosene binary solution as a

PDF] EXTRACTION OF ZN, MN AND CO FROM ZN-MN-CO-CD-NI CONTAINING

Solvent Extraction of Nickel and Zinc from Nitric Acid Solution Using D2EHPA: Experimental and Modeling

Developing a Thermodynamical Method for Prediction of Activity Coefficient of TBP Dissolved in Kerosene
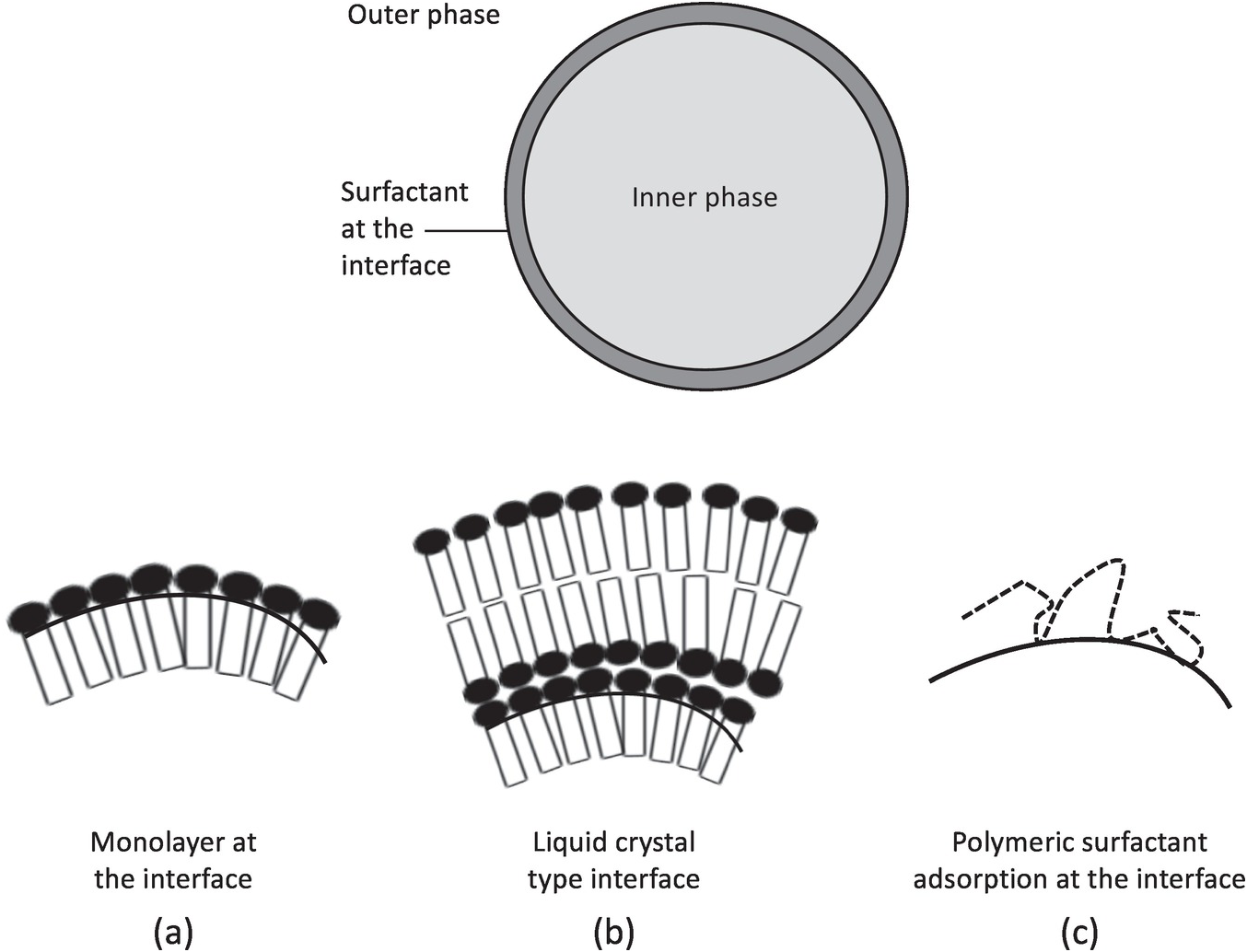
Membrane-Based and Emulsion-Based Intensifications (Chapter 4) - Intensification of Liquid–Liquid Processes

PDF] EXTRACTION OF ZN, MN AND CO FROM ZN-MN-CO-CD-NI CONTAINING

E. ALAMDARI, Professor (Associate), PhD

Lithium Recovery from Brines Including Seawater, Salt Lake Brine, Underground Water and Geothermal Water

Chinese Journal of Chemical Engineering
- Compressibility Factor from Redlick-Kwong Equations

- 1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts

- Compressor and jet vacuum system:, by Maryambotshekan

- Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT

- Solved RT B 2. The compressiblity factor for a gas is
- Barcode Berlin Singlet Harness Maximus 91966 C-302 red-black

- Great Ladies Mesh Underwear - Girl Fancy Bow Knot Briefs Cute Panties – Deals DejaVu

- L Pure Cotton Topsheet Pads For Women, Extra Long Overnight Pads, Maxi Pads

- Miss Selfridge satin frill detail mini slip dress in black
- Ellos Women's Plus Size Modern Stretch Chino Pants, 14 - New Khaki : Target


