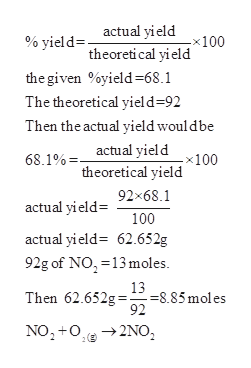
42g of N₂ react with excess of O₂ to produce NO. Amount of NO

By A Mystery Man Writer
Share your videos with friends, family, and the world

Answered: Gaseous ammonia chemically reacts with…

UMAIR KHAN ACADEMY

Limiting Reaction Calculations Practice Flashcards
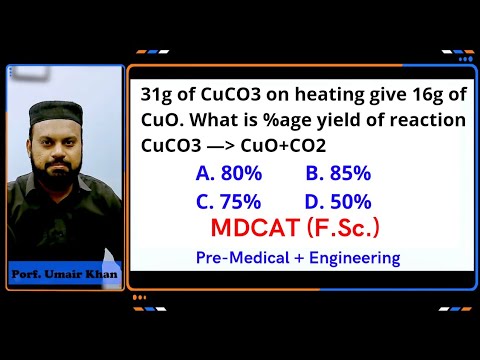
31g of CuCO3 on heating give 16g of CuO. what is %age yield of reaction. 80% 85% 75% 50%
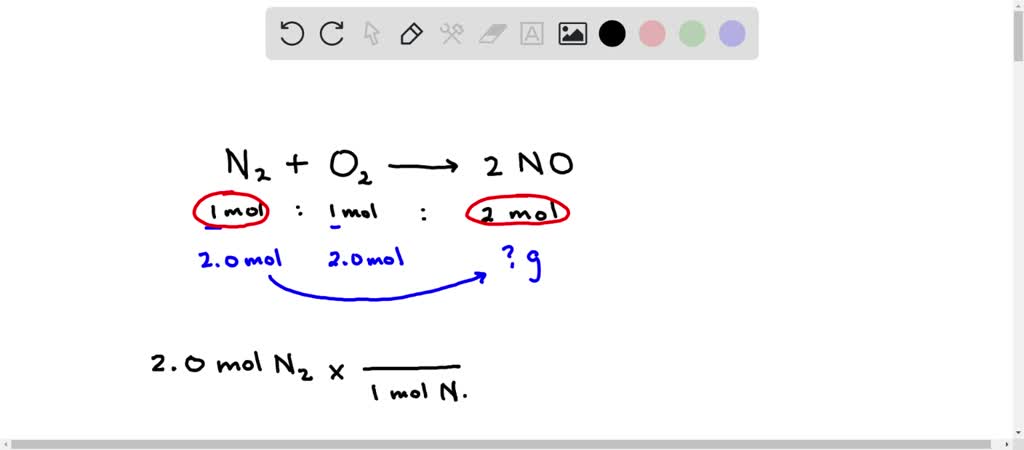
SOLVED: Given the balanced reaction: N2 + O2 → 2NO. How many grams of NO are produced from the reaction of 2.0 mol of N2 with 2.0 mol of O2? Select one

Answered: LIMITING REAGENTS TO2/S21 Based on the…

UMAIR KHAN ACADEMY

Solved Consider the reaction N2(g) +202(g) + 2NO2(9). The

42g of N₂ react with excess of O₂ to produce NO. Amount of NO formed is a.60g b.32g c.45g d.90g
Answered: How many moles of O₂ would be required…

Mole Concept PDF, PDF, Mole (Unit)
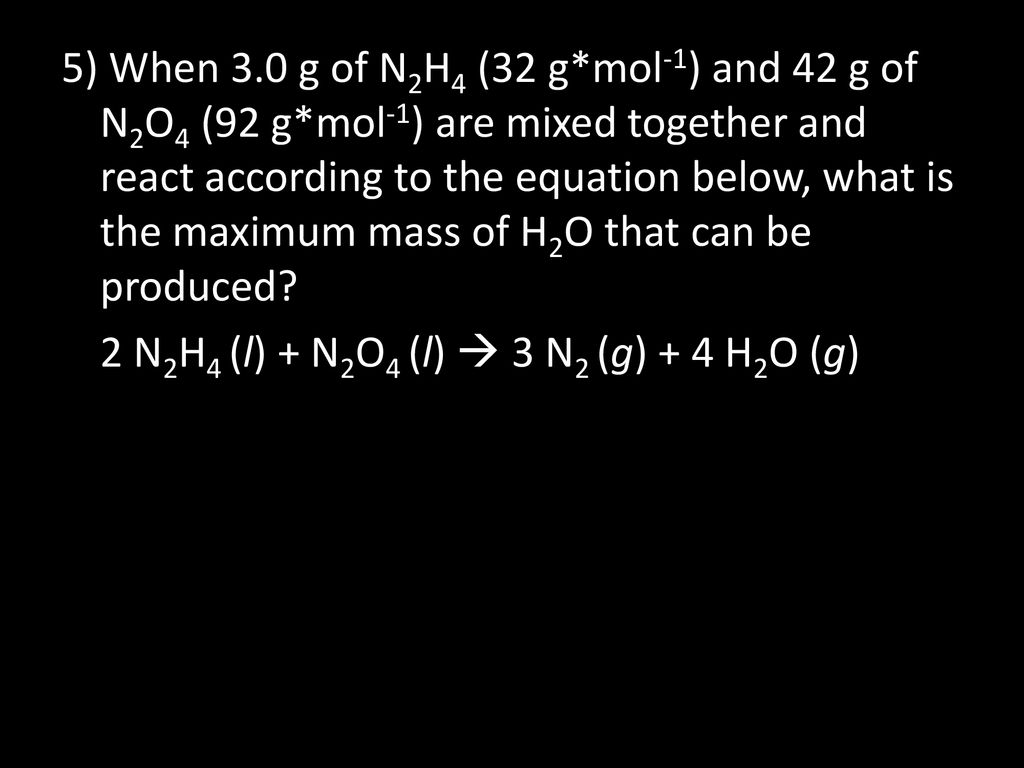
AP Chemistry Unit 2 Review: Choose your destiny - ppt download
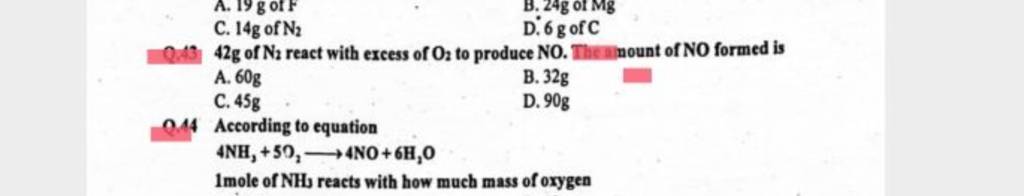
42 g of N2 react with excess of O2 to produce NO. Theninount of NO form..
- SNICKERS 42G MARACUJA

- Laroscobine Palladium E-UF PN Vitamin C 42g & Collagen 18g - Gold

- For Acer Aspire 3 A315-42 A315-42G A315-54 A315-54K N19C1 Laptop keyboard cover C shell 15.6 Inch Red/Black - AliExpress

- SIKYONA - SIKYON Sicyone, Sikyonie c. 450-425 AC. (7,5mm, 0,42g, 12h)

- MEUS Racing 1.2-inch Beadlock Wheels Brass Rim+ Aluminum 1.0(plus





