10.9: Real Gases - Deviations from Ideal Behavior - Chemistry LibreTexts
By A Mystery Man Writer
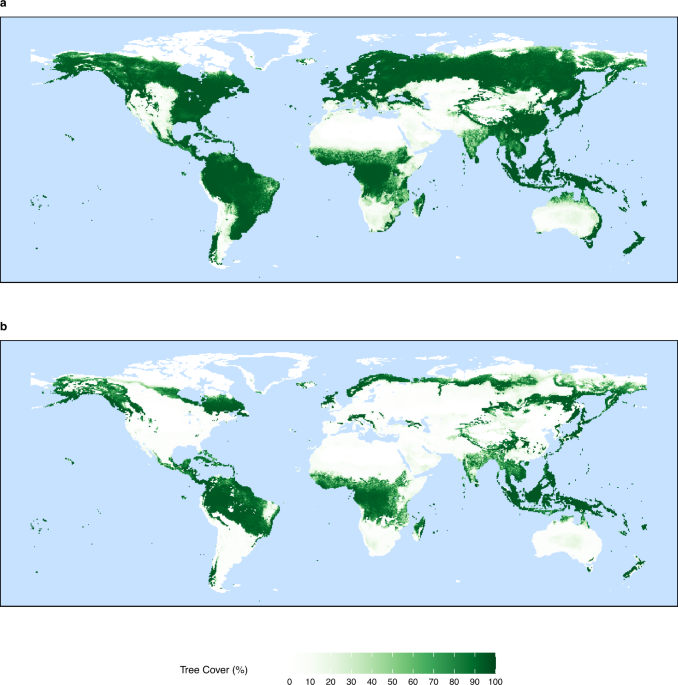
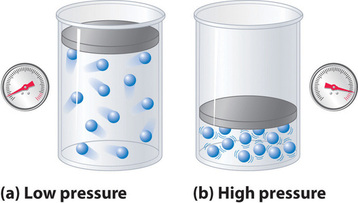
No real gas exhibits ideal gas behavior, although many real gases approximate it over a range of conditions. Gases most closely approximate ideal gas behavior at high temperatures and low pressures. …
No real gas exhibits ideal gas behavior, although many real gases approximate it over a range of conditions. Gases most closely approximate ideal gas behavior at high temperatures and low pressures. Deviations from ideal gas law behavior can be described by the van der Waals equation, which includes empirical constants to correct for the actual volume of the gaseous molecules and quantify the reduction in pressure due to intermolecular attractive forces.

Real gases: Deviations from ideal behavior, AP Chemistry

Chemistry For Engineering PDF, PDF, Atoms

Human Chemistry I PDF, PDF, Ionic Bonding

Real gases: Deviations from ideal behavior, AP Chemistry
How to predict which of the given gases is more non-ideal - Quora

Real gases: Deviations from ideal behavior, AP Chemistry

Thermodynamic & Chemical Equil PDF, PDF, Heat
What volume will 2.5mol of a gas occupy at 283K and at a pressure of 300torr under ideal conditions? (He also says 3 significant digits and ' (R=62.36L * torr/ (mol *

Real Gases: Deviations from Ideal Behavior - ppt download

Real gases: Deviations from ideal behavior, AP Chemistry

Real gases: Deviations from ideal behavior, AP Chemistry
How to predict which of the given gases is more non-ideal - Quora
- Under Armour Solid High Top Athletic Shoes for Women for sale

- How to Lower Cortisol: Foods and Activities to Try
:max_bytes(150000):strip_icc()/Health-Ways-to-Lower-Cortisol_Horiz-3b2f5e9ccace4c20b1416d36c0f93914.jpg)
- Diminishing returns on real estate threaten Chinese economic growth

- Logan Paul's Energy Drink Under Scrutiny Over High Caffeine

- Increased fire activity under high atmospheric oxygen concentrations is compatible with the presence of forests