Solved A 45-g block of copper at −12∘C is added to 120 g of
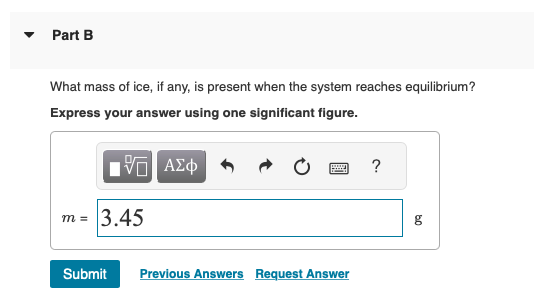
By A Mystery Man Writer
Answer to Solved A 45-g block of copper at −12∘C is added to 120 g of

What will be the equilibrium temperature when a 245-g block
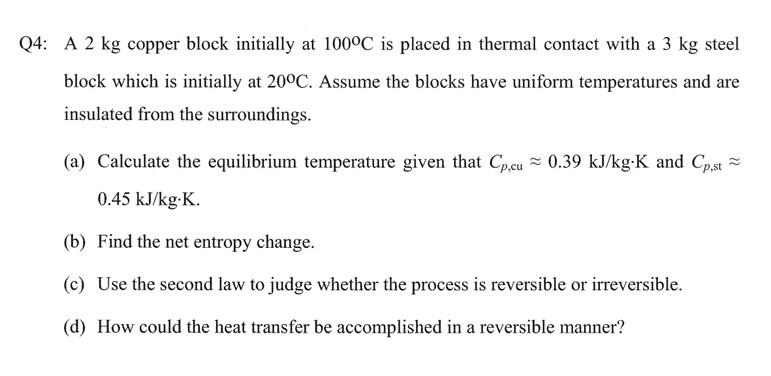
Solved A 2 kg copper block initially at 100∘C is placed in
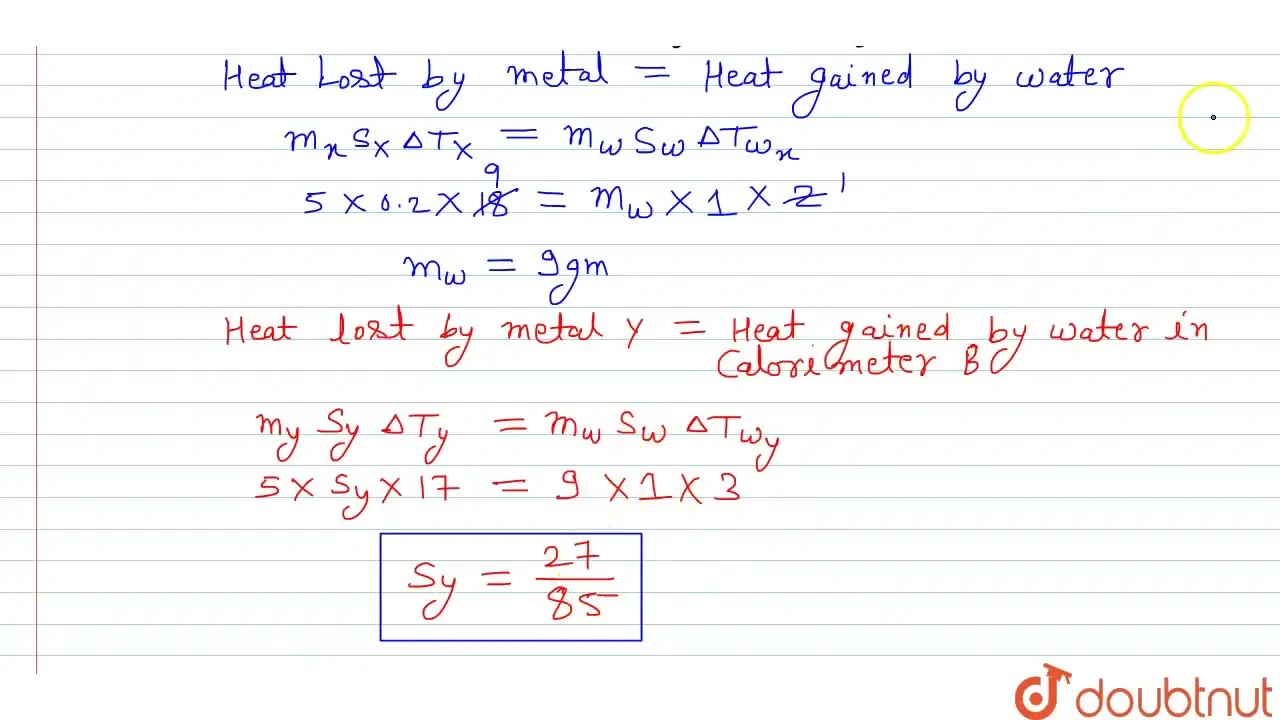
Two identical calorimeters A and B contain an equal quantity of water

200 g of hot water at 80^@C is added to 300 g of cold water at 10^@C.

A long constant wire is butt welded to the surface of a large copper block, forming a thermocouple junction. The wire behaves as a fin, permitting heat to flow from the surface

Energies, Free Full-Text

When a block of metal of specific heat 0.1 cal//g//^@C and weighing 11

Steam at 100°C is added to ice at 0°C. (a) Find the amount of ice melted and the final temperature w
✓ Solved: The heat capacity of a bomb calorimeter was determined by burning 6.79 g of methane (energy

⏩SOLVED:A coffee-cup calorimeter is used to determine the specific…

What will be the equilibrium temperature when a 245-g block
- VEKDONE Women Bras Clearance Sale Front Closure Bras for Women Comfort Push Up Bra No Underwire Padded Wireless Supportive Adjustable Bralette Bra

- Silicone Breast Forms E Cup with Liquid Silicone Silicone Breast

- COLLBATH 4pcs Elastic Adjustment Strap Waistband Elastic Underwear

- Women Full Body Shaper Compression Tummy Belly Shapewear Bodysuit

- Telová podprsenka bez ramienok Wonderbra