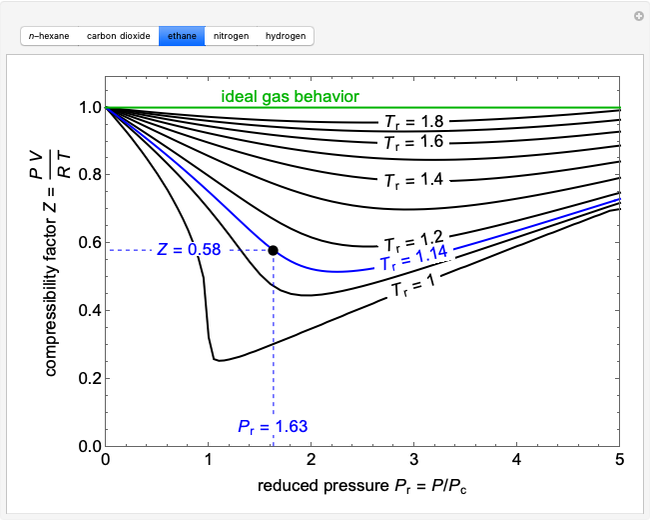
The compressibility factor Z a low-pressure range of all gases

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:the compressibility factor z at a lowpressure range of all gases except hydrogen is
Click here👆to get an answer to your question ✍️ The compressibility factor Z a low-pressure range of all gases except hydrogen is-Z-1- displaystylefrac-a-V-m-RT-Z-1-displaystylefrac-a-V-m-RT-Z-1-displaystylefrac-Pb-RT-Z - - 1 - displaystylefrac-Pb-RT-
The van der Waals equation for real gases is -P-aVm2-Vm-x2212-b-RT

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

Objectives_template

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

Compressibility Factor Calculator - File Exchange - MATLAB Central

Compressibility Factor Z Important Concepts and Tips for JEE Main
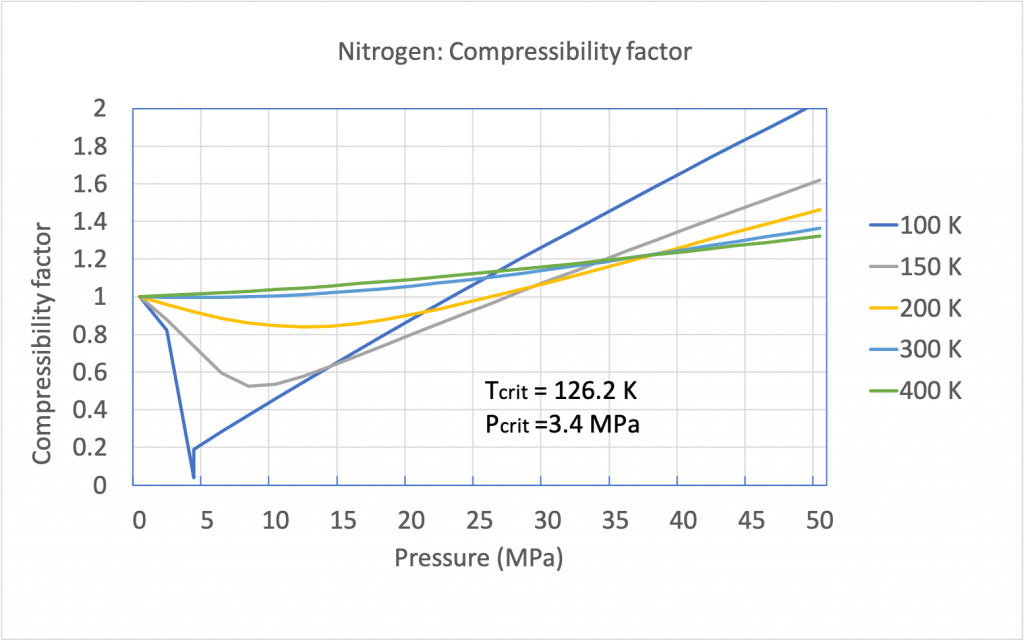
3.3: Real gas and compressibility factor - Engineering LibreTexts

2. U 0.52, 0.68, 0.74 At low pressure, the comprensibility factor is given as (1) - RTV Pb RT 12 12 Photo (3) 1+ TV Pb RT 3. 10 mole of an ideal gas 27°C ernands

Compressibility Factor Z

If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as
- Bunny Plush Animal With Mini Plush Bunny Stuffed Animal Toy - 2pc - Cloud Island™ : Target
- WWE legend Triple H announces in-ring retirement after suffering

- How To Choose Between Short and Long Riding Boots
- Womens Swimsuits Women Sport Bra Flower Printed Beach Beachwear Swim Bandeau Bandage Bikini Set Push Up Swimwear Bathing Suit Two Pieces Bikini Set

- Sound Control Singing Bird Red Bird Electric Voice-Activated Bird Pets – Devon Children Store