An ideal gas initially P_i ,V_i , and T_i is taken through a cycle as shown in Figure. (a) Find the net work done on the gas per cycle 1.00 mol of

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:an ideal gas initially at pi vi and ti is taken through a cycle
Click here👆to get an answer to your question ✍️ An ideal gas initially P-i -V-i - and T-i is taken through a cycle as shown in Figure- -a- Find the net work done on the gas per cycle 1-00 mol of gas initially 0-0C- -b- What is the net energy added by heat to the gas per cycle

A monoatomic ideal gas is taken through the cycle A ightarrow B ightarrow C ightarrow A as shown in the figure. Express all the answers below in terms of p_o and V_o.
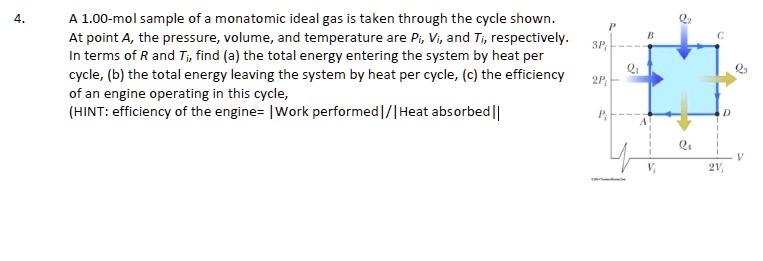
SOLVED: A 1.00-mol sample of monatomic ideal gas is taken through the cycle shown At point A, the pressure volume and temperature are Pi, Vi, and Ti respectively: terms of R and

An ideal gas initially at P_i, V_i and T_i is taken through a cycle as shown below. Let the factor n = 3.7. a. Find the net work done on the gas

One mole of an ideal monatomic gas is taken round the cyclic process ABCA as shown in figure.

The figure shows a reversible cycle through which

Thermodynamics problems

에너지
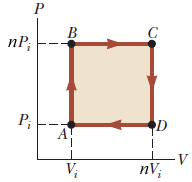
Solved An ideal gas initially at Pi, Vi, and Ti is taken

which is not really negligible with respect to unity Therefore the complete

Problems of definition and geographical differentiation of the subarctic with special regard to northern Europe
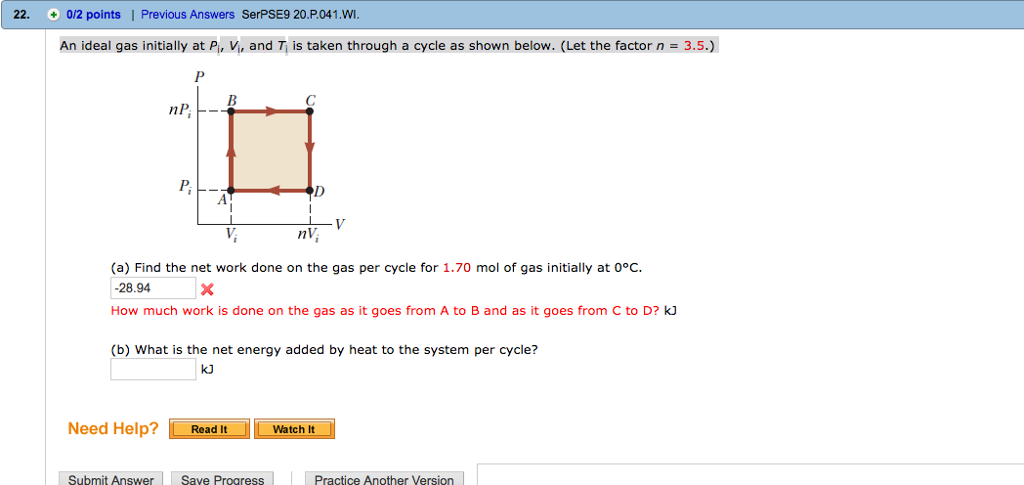
Solved An ideal gas initially at Pi, Vi, and Ti is taken
- LIPOELASTIC® PI Ideal Variant - Seamless Elastic Cups (XS, Black) : : Fashion

- a) Ideal phase inverter. (b) Configuration of a DSPSL PI (top strip in

- ЛИПОЕЛАСТИК PI IDEAL СЛЕДОПЕРАТИВЕН СУТИЕН (LIPOELASTIC PI IDEAL POST - SURGICAL BRA), цена и информация

- Apple Pi Day T Shirt Great Gift Idea For Math Lover – Teezou Store

- Lipoelastic PI Perfect Post Surgery Bra - Natural

- Jolie Grande bag by Naledi Copenhagen – Naledi Copenhagen Official

- Curvy Couture SHEER MESH PUSH UP BLACK HUE buy for the best price

- Nike Pro Mesh Logo Tights, Nordstrom

- Olive Green Workout Shorts with Compression Pants - Men's

- Draped Bustier Corset Midi Dress Red - Luxe Midi Dresses and Luxe Party Dresses
