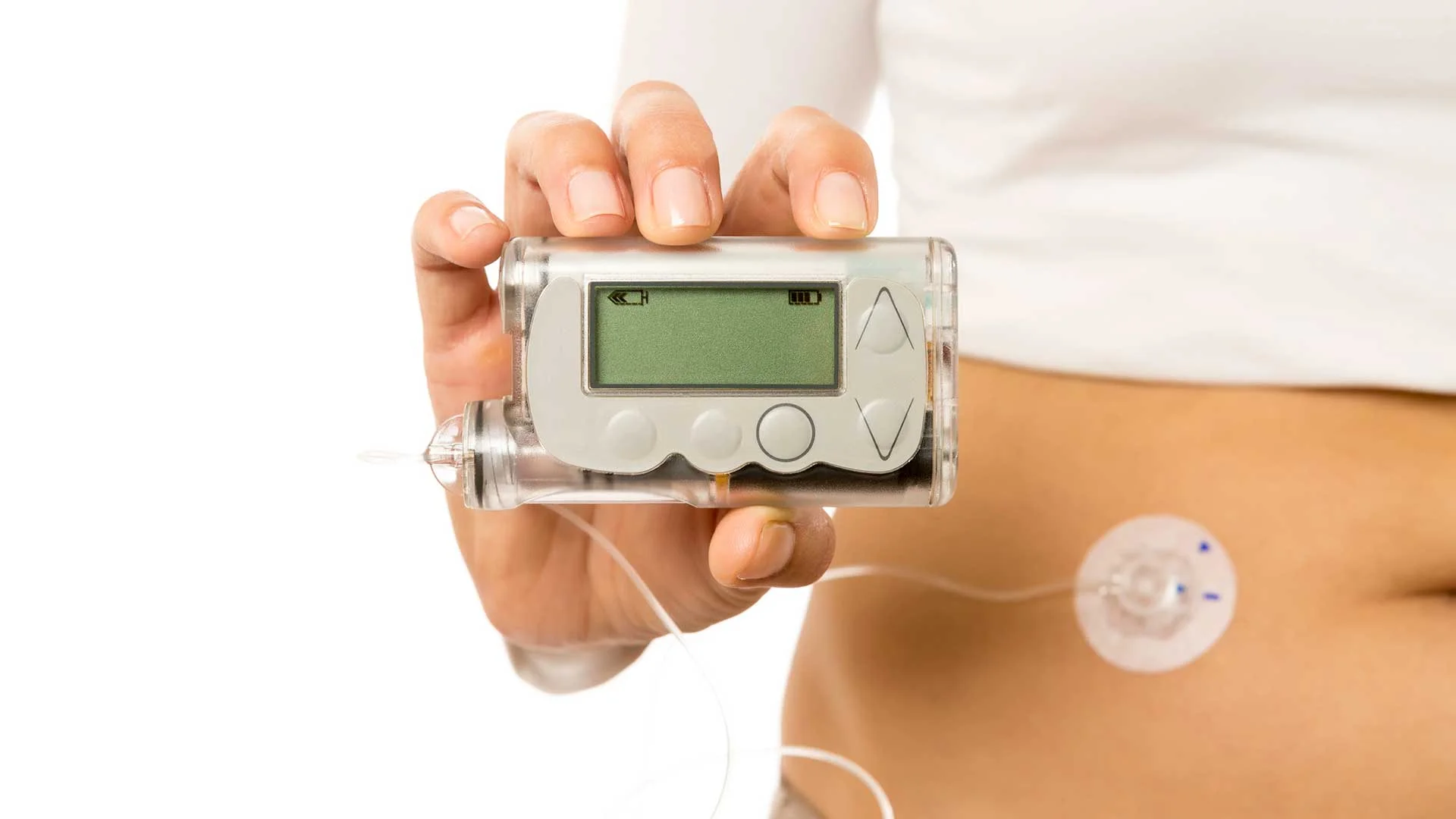
PharmaSens seeks FDA approval for new insulin pump

By A Mystery Man Writer
PharmaSens has submitted an application to obtain FDA approval for its ‘niia essential’ insulin patch pump system.

FDA Approval for Tandem's Basal-IQ Predictive Low Suspend for the

FDA Advisory No. 2020-1466

Wearable Tech Archives - Medical Device Network

Kazakhstan Archives - Medical Device Network

Empowering Diabetes Management: The Role of Insulin Pumps in

Tissue sealants and adhesives market in New Zealand

Animas® Vibe™ Insulin Pump and Continuous Glucose Monitoring

Regulation Archives - Medical Device Network

Snapshot: Changing Regulations in the European Medical Devices

Report: Medtronic in negotiations to buy Israel-based insulin pump

Metabolic Disorders Archives - Medical Device Network

ㅤ

Animas® Vibe™ Insulin Pump and Continuous Glucose Monitoring

Kazakhstan Archives - Medical Device Network

PharmaSens: Simplifying diabetes management through technology
- Freya Bras - Gorgeous Freya Bra Designs for Fuller Busts - Curvy

- Chemise

- CADEX DEFENCE KRAKEN Multi-Cal Rifle, 338 Lapua, 27.00

- Wholesale SILKIES Gaff Underwear - Thong - Tucking - Crossdressing

- QLEICOM Womens Swimsuits Tummy Control Plus Size Swimsuit Coverup One-Piece Padded Plus Size Overlay Print Bikini Swimsuit Pink XXL