What mass of carbon is present in 44g of carbon dioxide? - Quora
By A Mystery Man Writer

Calculate the mass of CO2 which contains same number of molecules present in 40g of O2.

What is the mass of carbon dioxide which contains the same number of molecules as are contained in 40 g of oxygen?55 g40 g32 g44 g
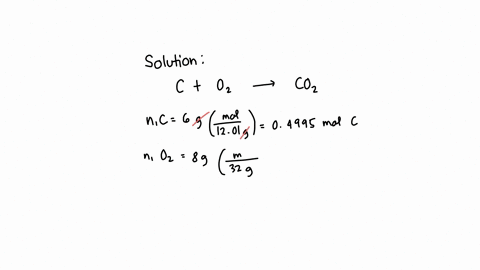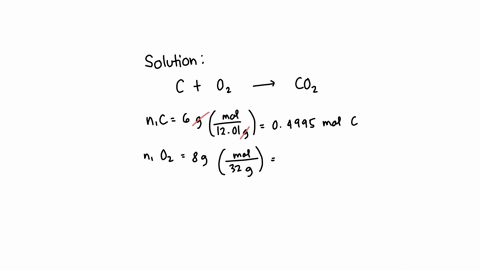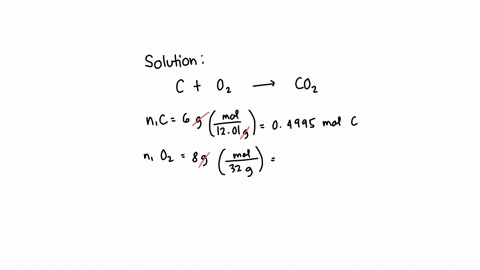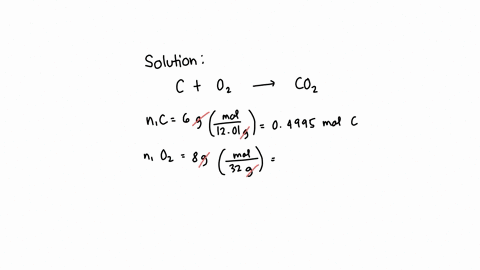
SOLVED: What is the mass of carbon present in 44g of carbon dioxide.
What mass of carbon dioxide would we have if we had 1.5 litres at RTP? - Quora
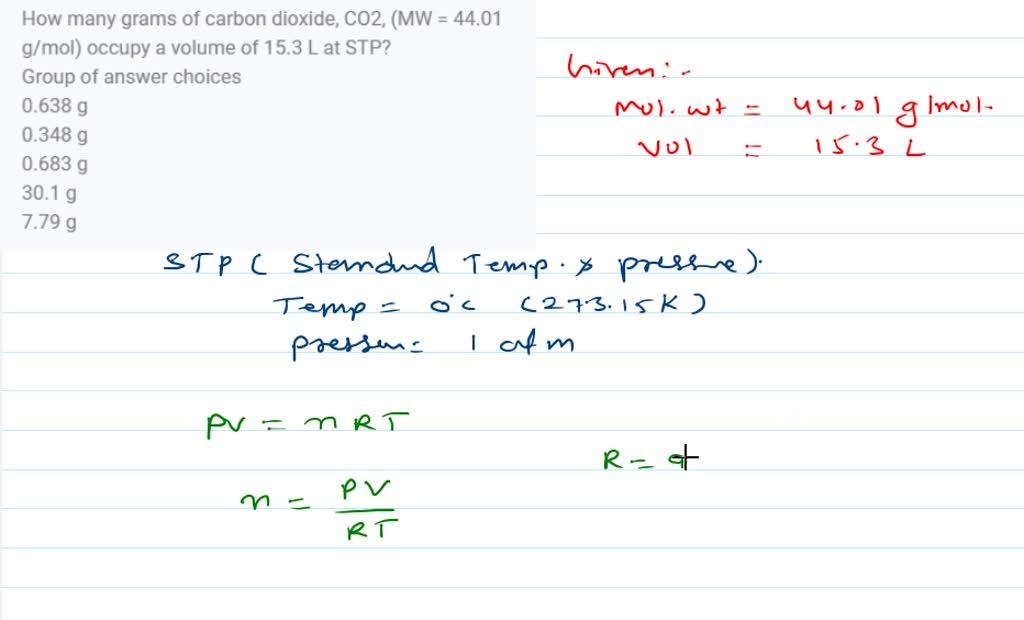
SOLVED: How many grams of carbon dioxide, CO2, (MW = 44.01 g/mol) occupy a volume of 15.3 L at STP? Group of answer choices 0.638 g 0.348 g 0.683 g 30.1 g 7.79 g
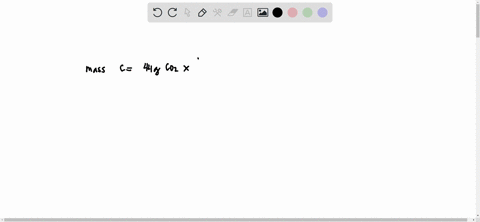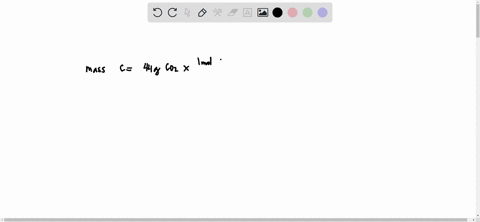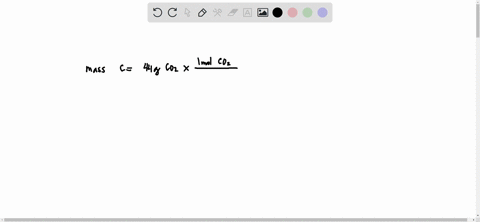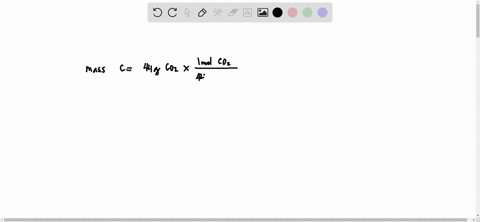
SOLVED: What is the mass of carbon present in 44g of carbon dioxide.
What mass of carbon is present in 44g of carbon dioxide? - Quora
What is the volume of 5.00 g of carbon dioxide gas at STP using GRESS? - Quora

How to Find the Mass of One Molecule of Carbon dioxide (CO2)
- Grumman G-44 Widgeon - National Museum of World War II Aviation

- The Perfect Seaplane?!! / FT Grumman G- 44 Widgeon

- Botana Sabritas Paketaxo Mezcladito chile, sal, limón y especias 44 g

- Bojo Biquini Ref.0800 Par Tam G /44

- Quadro de Distribuição 44 Din Embutir Sem Barramento G Luna Eletrosul Materiais Elétricos e Hidráulicos Embutir S/ Barramento





