What is the compressibility factor (Z) for 0.02 mole of a van der

By A Mystery Man Writer

6.3: Van der Waals and Other Gases - Physics LibreTexts
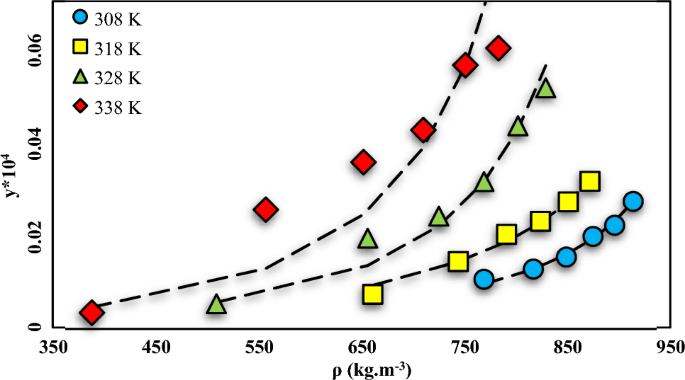
A machine learning approach for thermodynamic modeling of the statically measured solubility of nilotinib hydrochloride monohydrate (anti-cancer drug) in supercritical CO2

SOLVED: Find the compressibility factor Z for a real gas with the van der Waals equation of state when: a) There are no attractive forces within gas molecules? b) There are no

Determine Compressibility of Gases

Sheet - 01 - Real Gas, PDF, Gases

Real Gases, PDF, Gases

DPP No. : 21 Total Marks : 40 Max. Time : 10mln Single choice Objective (..

63. What is the compressibility factor (2) 0.02 mole of a van der Waals' gas pressure of 0.1 atm. Assume the size of gas molecules is negligible. Given : RT = 20

Compressibility factor - Wikipedia

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Energies, Free Full-Text
- Thermodynamics - 3-7 Ideal Gas Equation with compressibility factor

- Compressibility factor (gases) - Knowino

- Compressibility Factor Calculator - Community
- 1.7: Connecting the van der Waals and the viral equations: the

- Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of

- Linzee Marie on X: Ok so I had an itchy boob earlier, just noticed this🙈 not normal right #itchy #boob #burstbloodvessels #odd #helllpp / X
- VAVA VOOM #14 Groovy Goose issue

- LAEMILIA Women Low Rise Jeans Bootcut Premium Stretchy Flare Denim

- Women's High-Rise Ankle Jogger Pants - A New Day Cream XL 1 ct

- Lucky Brand H212 Classic Straight 100% Cotton Denim Jeans. Medium
