What is the change in internal energy (in J) of a system that

By A Mystery Man Writer
I found an increase of 3100J Have a look

General Chemistry 2 Worksheet 4. Thermochemistry

OneClass: Calculate the change in internal energy (Delta E) for a system that is absorbing 35.8 kJ o
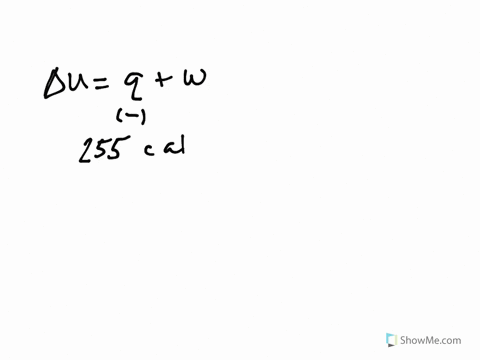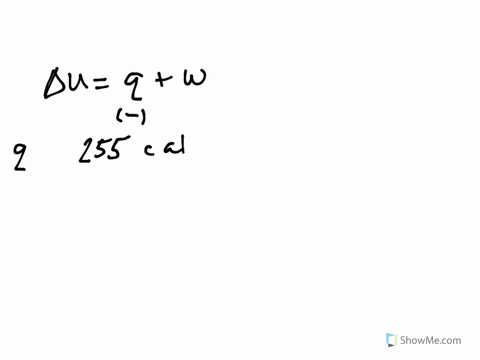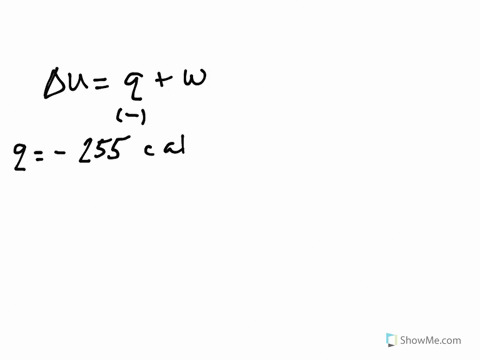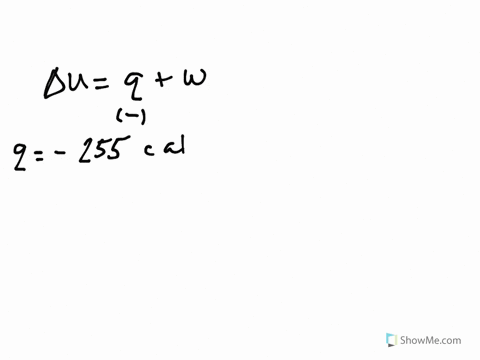
⏩SOLVED:A system releases 255 cal of heat to the surroundings and

Solved Be sure to answer all parts. What is the change in
Solved What is the change in internal energy (in J) of a

Ch6.1 The Nature of Energy (hustle!) - ppt download

How to calculate ΔE when the system absorbs 250 J of heat energy
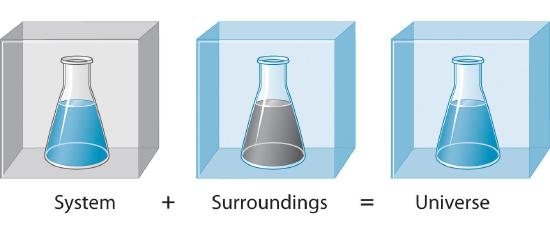
6.3: The First Law of Thermodynamics: Internal Energy - Chemistry LibreTexts
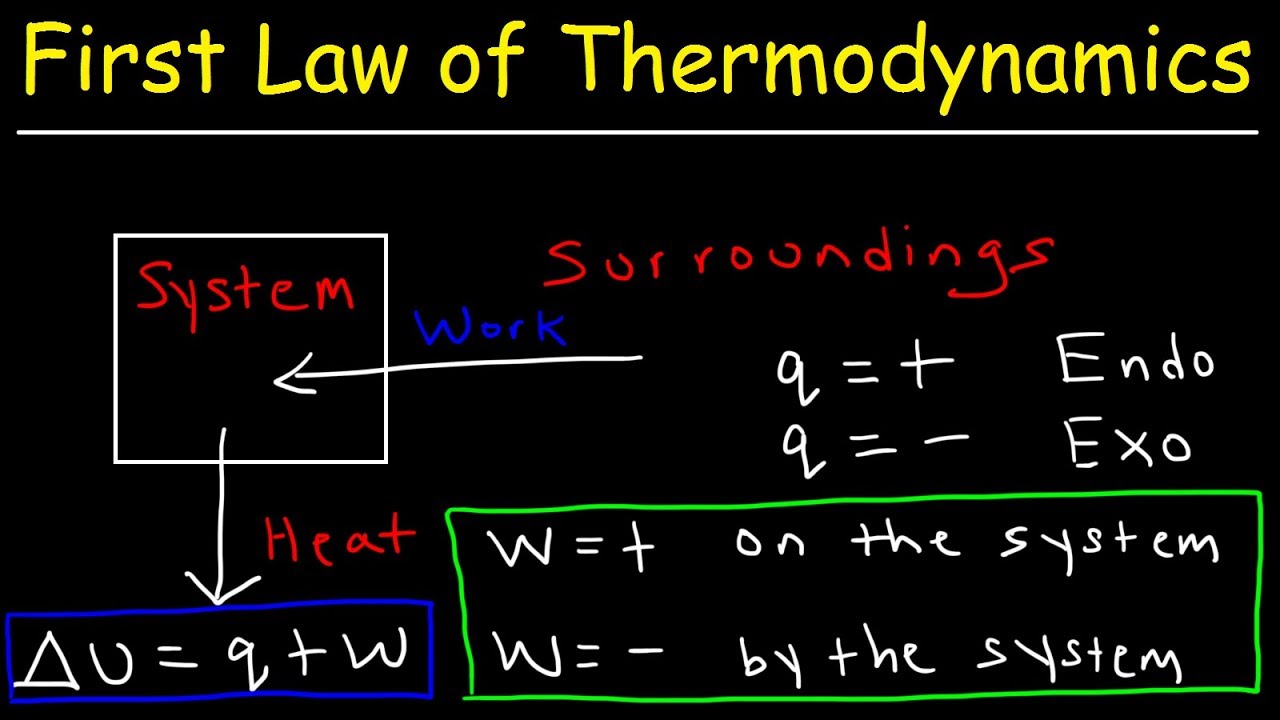
CaptionSync Smart Player™

Solved What is the change in internal energy, ΔU, in Joules

What is the change in internal energy ΔU, for a system that does 70 joules of work as it absorb
- V Ruffles Neck Viscose Cotton Dress Prom Cocktail Dresses Maxi

- What are the most visited social media platforms among Gen Z? - Comscore, Inc.

- Women Drawstring Pants, Fashion Women's Loose Sequin Stripe Printed Casual Pants Side Pocket Autumn (Green, S) at Women's Clothing store

- KECKS Women's Tops Shirts Sexy Tops for Women Drop Shoulder Button Front Belted Shirt Shirts for Women (Color : Apricot, Size : Medium) : Clothing, Shoes & Jewelry

- Buy ModBra Women Transparent Strapless Backless Invisible Clear Back Underwire Push Up Padded Bra(Fit Size 32B) (Pack of 2) Online at Best Prices in India - JioMart.
)



