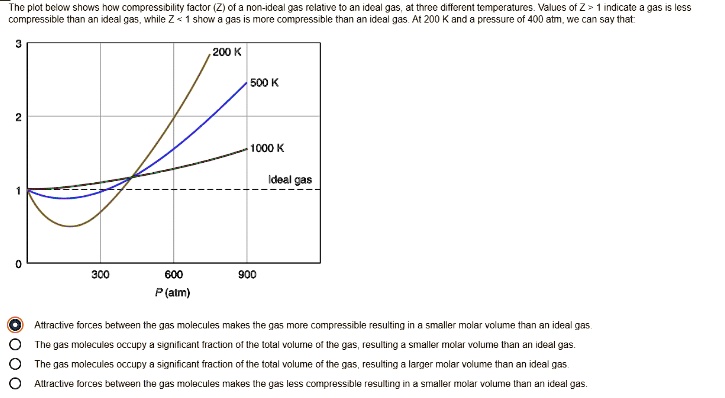
SOLVED: Plot bclon shcs now compressibility factor (Ziofa non-Idc? 935 relative to an ideal gas; J1 force differential Mocraiurc: Values of Z indicate compressibility and inan re any more compressible ideal gas
By A Mystery Man Writer
VIDEO ANSWER: We are going to see the difference between the liquid and the guest. The chemical entities are associated with bonds in gas. There are chemical entities away from each other. The guests here have a random motion system that is
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.

SOLVED: Plot bclon shcs now compressibility factor (Ziofa non-Idc? 935 relative to an ideal gas; J1 force differential Mocraiurc: Values of Z indicate compressibility and inan re any more compressible ideal gas
New explicit correlation for the compressibility factor of natural gas: linearized z-factor isotherms

Assertion: Compressibility factor `(Z)` for non ideal gases is always greater than `1`.

PV Compressibility factor Z= nRT is plotted against pressure : N. Ideal gas What is the correct order of liquefiability of the gases shown in the above graph? H
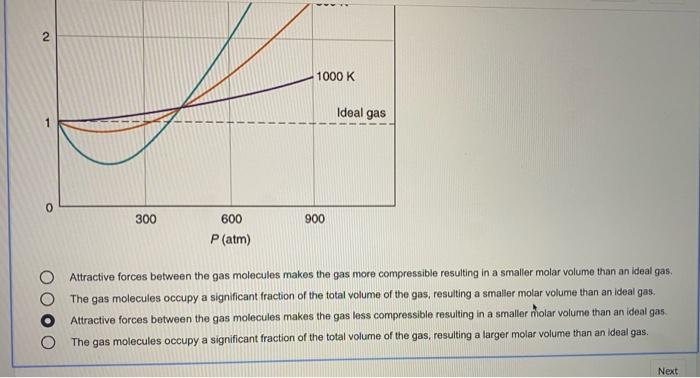
Solved 6 1 point The plot below shows how compressibility

of Z-factor Koch 75.(A) Assertion : Compressibility factor (Z) non-ideal gases is always greater than 1. Reason: Non-ideal gases always exert higher pressure than expected. (a) A (b) B (c) C 76. (

4.2: Real Gases (Deviations From Ideal Behavior) - Chemistry LibreTexts

Compressibility factor - Wikipedia

Assertion: Compressibility factor `(Z)` for non ideal gases is always greater than `1`.
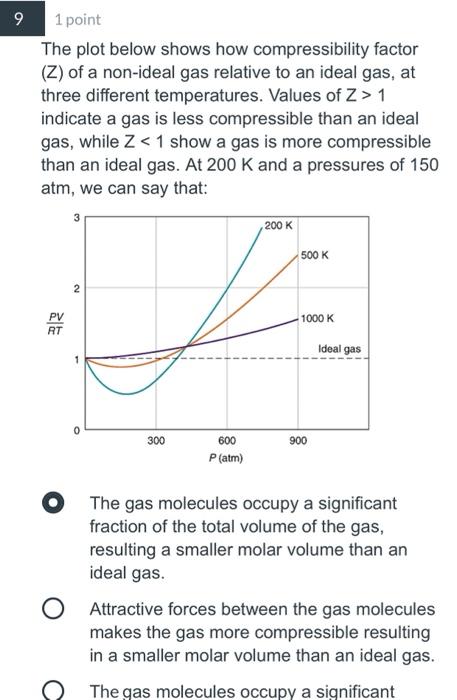
Solved 1 1 point If the root mean square speed of a gas

Ideal Gas Equation and COMPRESSIBILITY Factor in 11 Minutes!

Statement-1. Compressibility factor of non-ideal gases is always less than1. Statement-2. Non-id
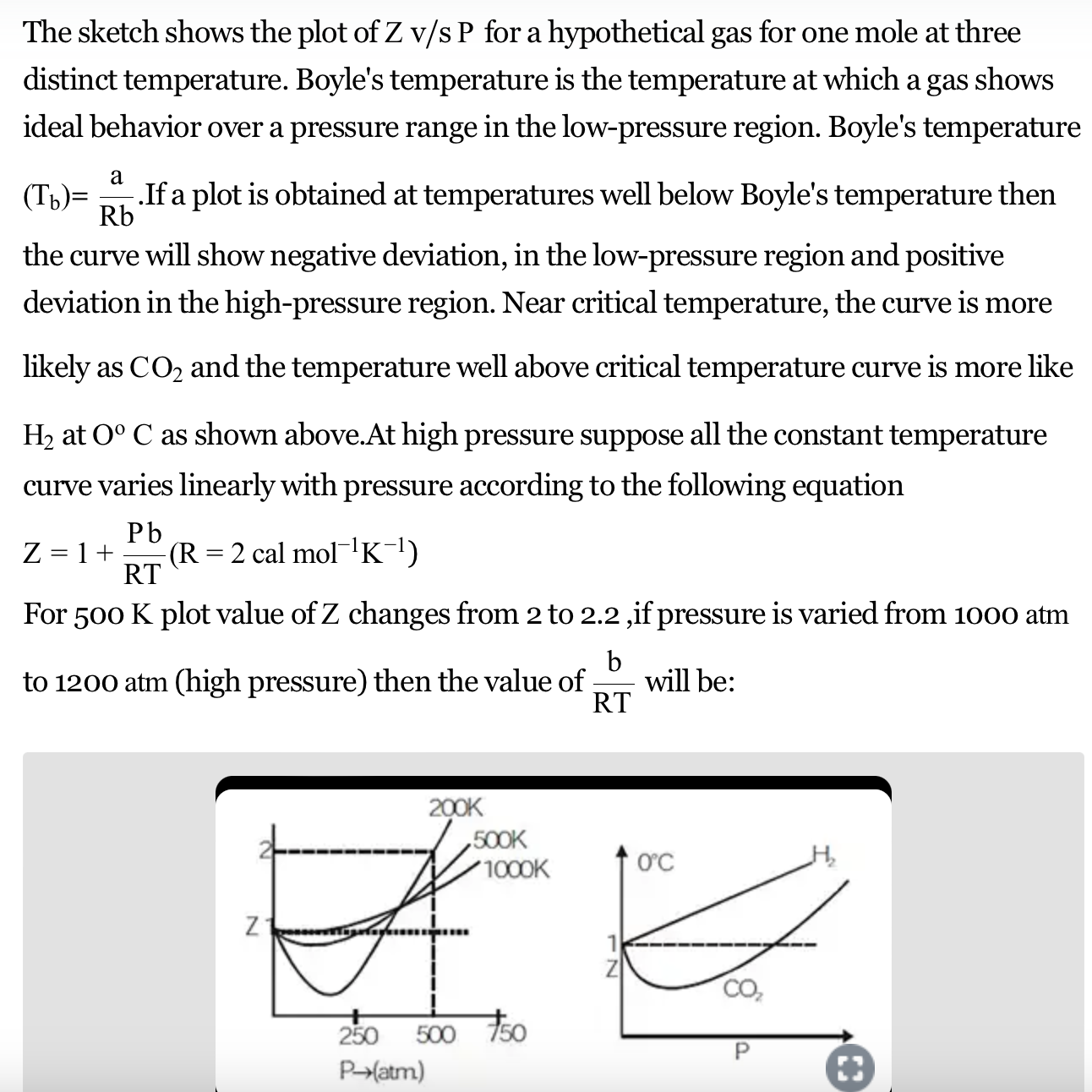
Solved The graph of compressibility factor (Z)v/sP for 1 mol

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson
- Compressibility Factor Z Important Concepts and Tips for JEE Main

- New explicit correlation for the compressibility factor of natural

- plotting - How to plot Compressibility factor Z vs Pressure P

- In the following compressibility factor Z vs pressure graph at 300

- Compressibility factor (Z) is plotted against pressure at different te

- Bodysuit Shapewear for Women Tummy Control Dress Backless Bodysuit Tops Body Shaper with Built-in Br (Color : Black, Size : L) (Natural XL) (Natural L) : : Clothing, Shoes & Accessories

- Cortland Intimates Lace Control Panty

- Buy Poomex® Men's Cotton Half Sleeve Vest (Pack of 3) (XS) White at

- Woman with a Whip in Her Hand Stock Image - Image of face, caucasian: 51593503

- Pack of 3 girls' White Blue Les Pockets stretch cotton knickers with a star pattern
