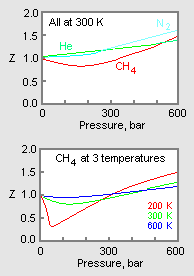
For $CO$, isotherm is of the type as shown. Near the point

By A Mystery Man Writer
For $CO$, isotherm is of the type as shown. Near the point compressibility factor $Z$ is?\n \n \n \n \n 1.$\\left( {1 + \\dfrac{b}{V}} \\right)$ 2.$\\left( {1 - \\dfrac{b}{V}} \\right)$3.$\\left( {1 + \\dfrac{a}{{RTV}}} \\right)$4.$\\lef
van der Waals isotherms of CH 4 near critical temperature. Shaded

NEET Chemistry Chapter Wise Mock Test - General Chemistry - CBSE Tuts
In the above Question, near the point B, compressibility factor Z is a

NEET Chemistry Chapter Wise Mock Test - General Chemistry - CBSE Tuts

Correction to: The effect of atomic point charges on adsorption

11111 Umu) 32 min 46. The ratio of van der Waals' constants a and b, has the dimension of lá atm L- ((b) L atm mol-' (c) L mol-1 (d) atm L

NEET Chemistry Chapter Wise Mock Test - General Chemistry - CBSE Tuts

Adsorption isotherm classi®cation as proposed by Giles et al
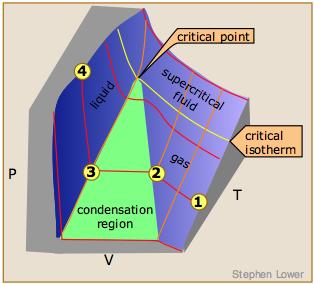
1.5: Condensation of Gases & the Critical State - Chemistry LibreTexts

NEET Chemistry Chapter Wise Mock Test - General Chemistry - CBSE Tuts
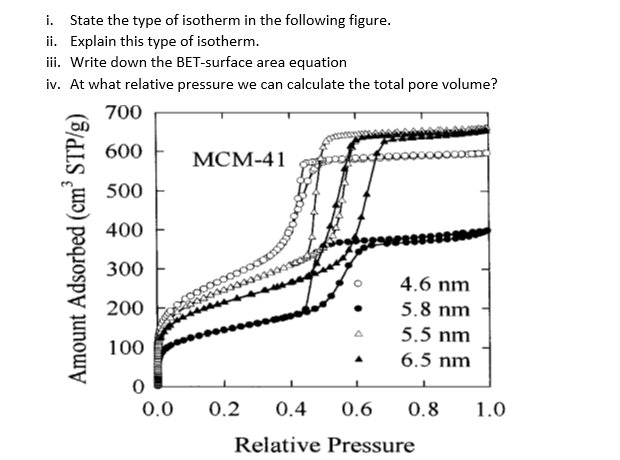
Solved i. State the type of isotherm in the following
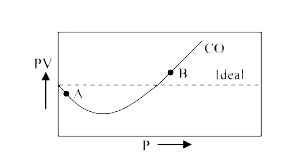
For CO, isotherm is of the type as shown: Near the point A, compr

Adsorption isotherm showing capillary condensation (Fig. 1, Ref

For CO, isotherm is of the type as shown:Near the point A, compressibility factor Z (for 1 mol o

Ask Answer - Chemistry - Recently Asked Questions for School Students
- Introduction to First-Trimester Point-of-Care Ultrasound - Point-of-Care Ultrasound Certification Academy
- Women's Merino Wool Leggings - Natural ❤️ menique

- A handsome hipster young man with formal suit sitting on a stool on an indoor party, looking away. photo – Sitting Image on Unsplash
- Kyodan Womens Soft Camo Jacquard Ultra High-Waist Leggings

- Broken Heart Syndrome - Health Beat

- Eddie Bauer Fleece Lined Pants Black Size 4 - $20 (77% Off Retail

- Snow Gear for Toddlers Jess Ann Kirby - Lifestyle, Fashion

- Kim Kardashian Gets Hello Kitty Cake for Daughter's 5th Birthday: Photo
:max_bytes(150000):strip_icc():focal(561x0:563x2)/chicago-west-kim-kardashian-hello-kitty-birthday-party-011623-14-2000-15394687540e453db2a2a71448d24874.jpg)
- 나이키 슈즈백 DM3978-010 블랙 브랜드 중고거래 플랫폼, 번개장터

- Leggings acampanados con aberturas de talle alto reciclados, ajuste elástico suave y mantecoso en 4 direcciones y pantalones acampanados con aberturas - México