200 g of a sample of limestone liberates 66 g of CO2 on heating
By A Mystery Man Writer
200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
200 g of a sample of limestone liberates 66 g of CO2 on heating- The percentage purity of CaCO3 in the limestone is Options-a- 95

400 g sample of limestone liberates 44 g carbon dioxide on heating. The p..

US20060276339A1 - Methods and compositions for increasing the efficacy of biologically-active ingredients - Google Patents

Thermochemical energy storage system development utilising limestone - ScienceDirect
6.5 g of an impure sample of limestone liberates2.2 g of CO2 on strong heating. The percentagepurity of CaCO3 in the sample is(1) 85.2
When a limestone of mass 150g was heated until it decomposed to CaO, only 63g of CaO were obtained. What is the percentage purity of the limestone? - Quora
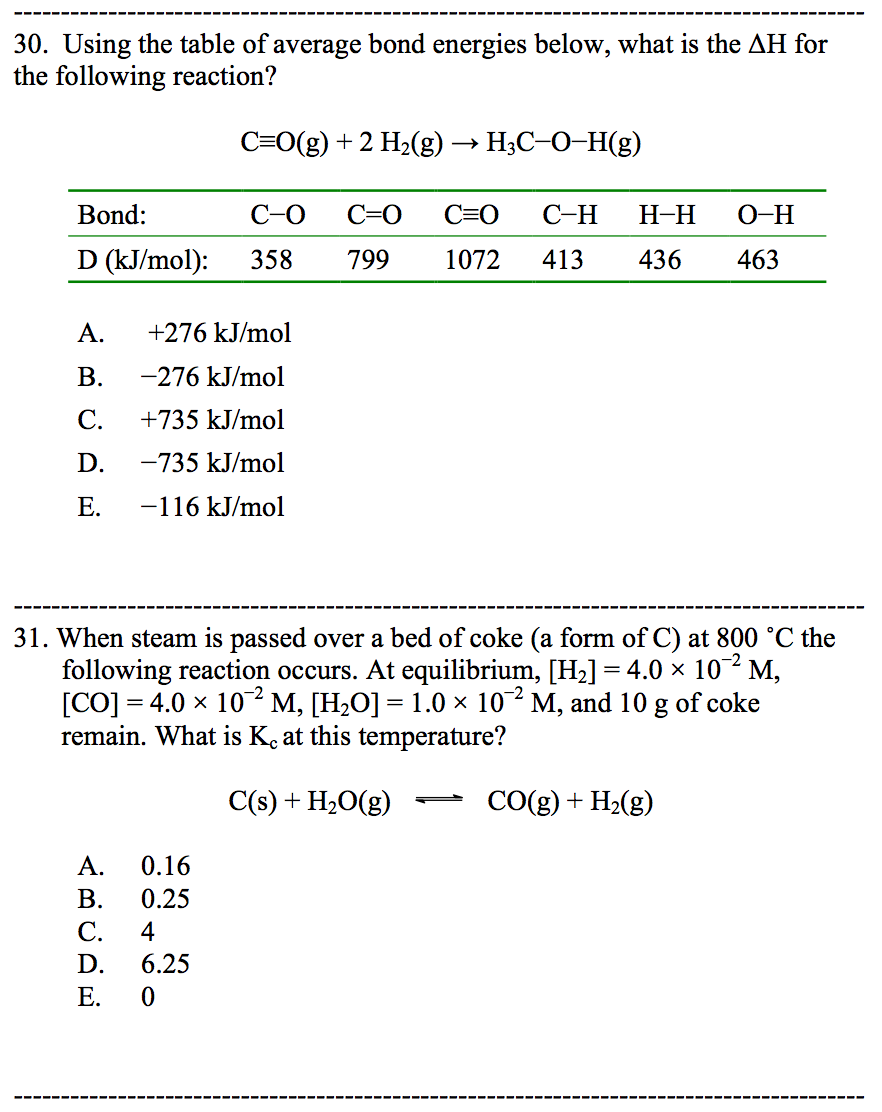
Solved Please help me solve the following questions below

Chapter 9

When 200 g of lime strongly heated , it undergoes thermal decomposition to form 112 g of lime and

Carbonate geochemistry and its role in geologic carbon storage - ScienceDirect

CHEMICAL REACTION AND EQUATIONS

AP Chem MC Practice ProblemsKey, PDF, Radioactive Decay
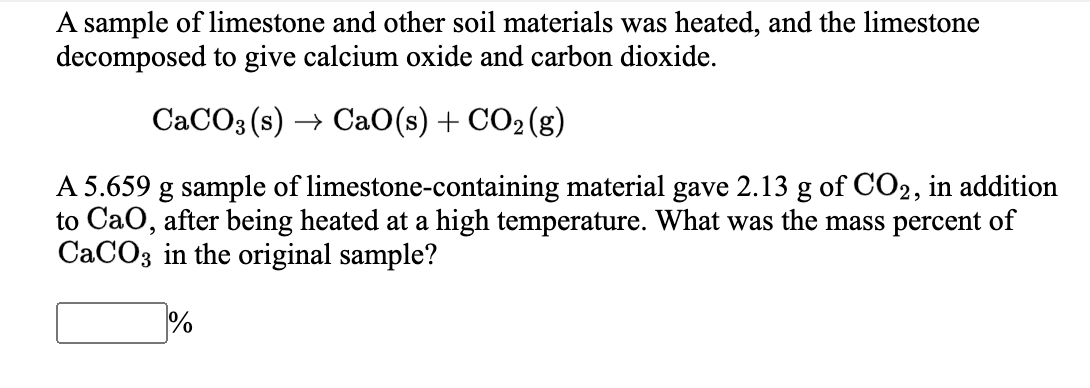
Solved A sample of limestone and other soil materials was

Carbonate geochemistry and its role in geologic carbon storage - ScienceDirect
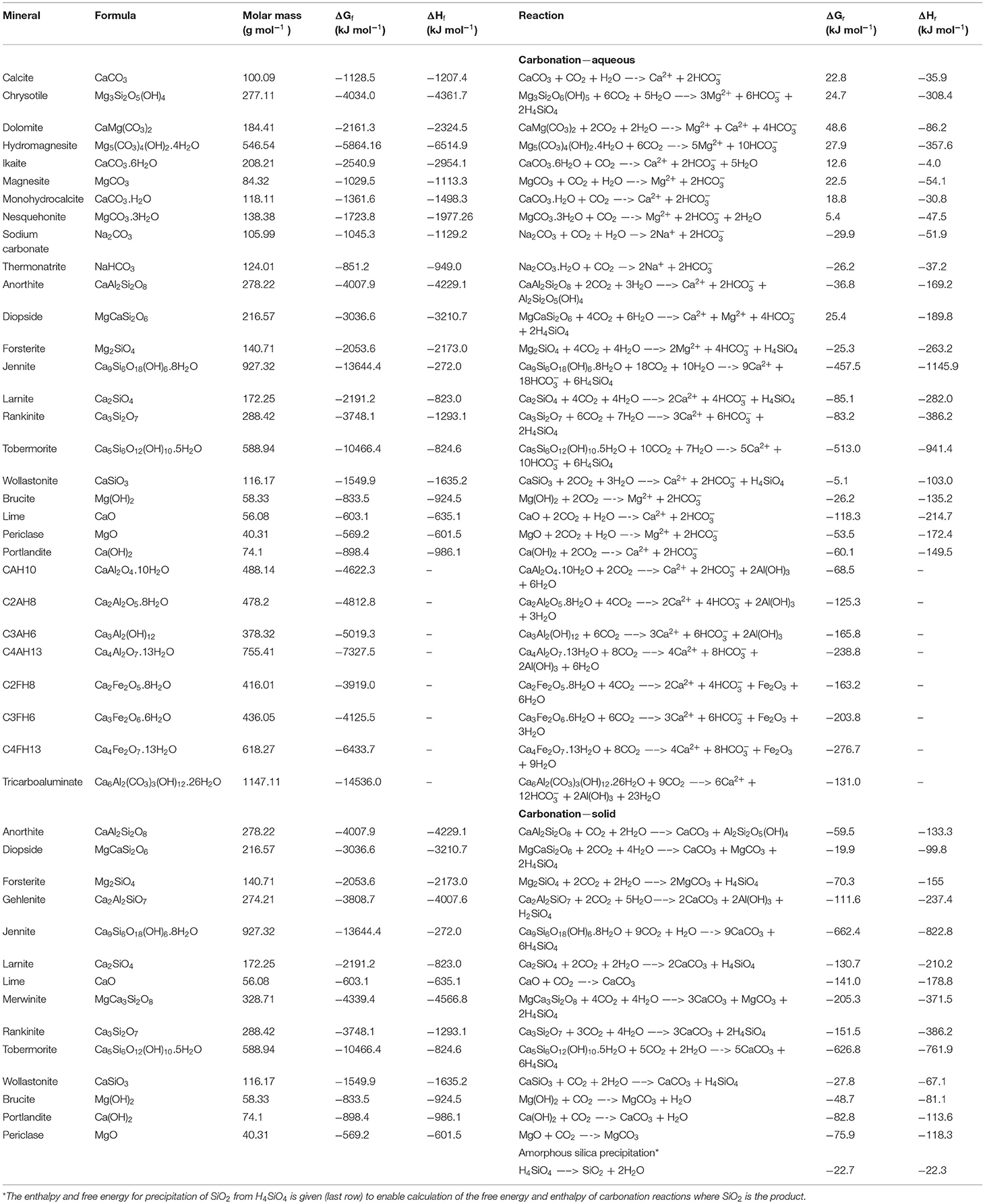
Frontiers Geochemical Negative Emissions Technologies: Part I. Review
- FRALDA DRY EVOLUTION MEGA G 44UN - Fralda Infantil Dry Evolution Mega G 44 Unidades - NAO INFORMADO

- Drogaria Santa Terezinha

- Fralda Descartável Infantil Cremer Magic Care G Pacote 44 Unidades - Supernosso

- Rocko Marinela 44 g - Tu Mini Súper

- CHI 44 Iron Guard Style & Stay Firm Hold Protecting Spray - CHI

- Agasalho Nike Sportswear Sport Essentials Masculino - Escorrega o Preço

- KIHOUT Deals Women's Ink Yoga Tie-Dye Pants Slim And Hip Lifting Exercise Bottom Pants

- Women's One Piece Swimsuits Full Coverage Womens Summer Beach Bikini Large Swimsuit Women's Swimwear One Piece Plus, Green, Small : : Clothing, Shoes & Accessories

- SAVVI WOMENS LARGE TAO TANK TOP EVERYDAY ACTIVE WEAR - BRAND NEW WITH TAGS SAVVY

- Invisalign Cancellation Fees, Digital Dentistry Blog