the compression factor one mole of a vander waals gas 0 C and 100

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:the compression factor for one mole of a vander waals gas at 0 c and
Click here👆to get an answer to your question ✍️ The compression factor one mole of a vander waals gas 0 C and 100 atm pressure is found to be 0-5

Van Der Waals Equation - an overview

What is the compressibility factor (Z) for 0.02 mole of a van der Waal
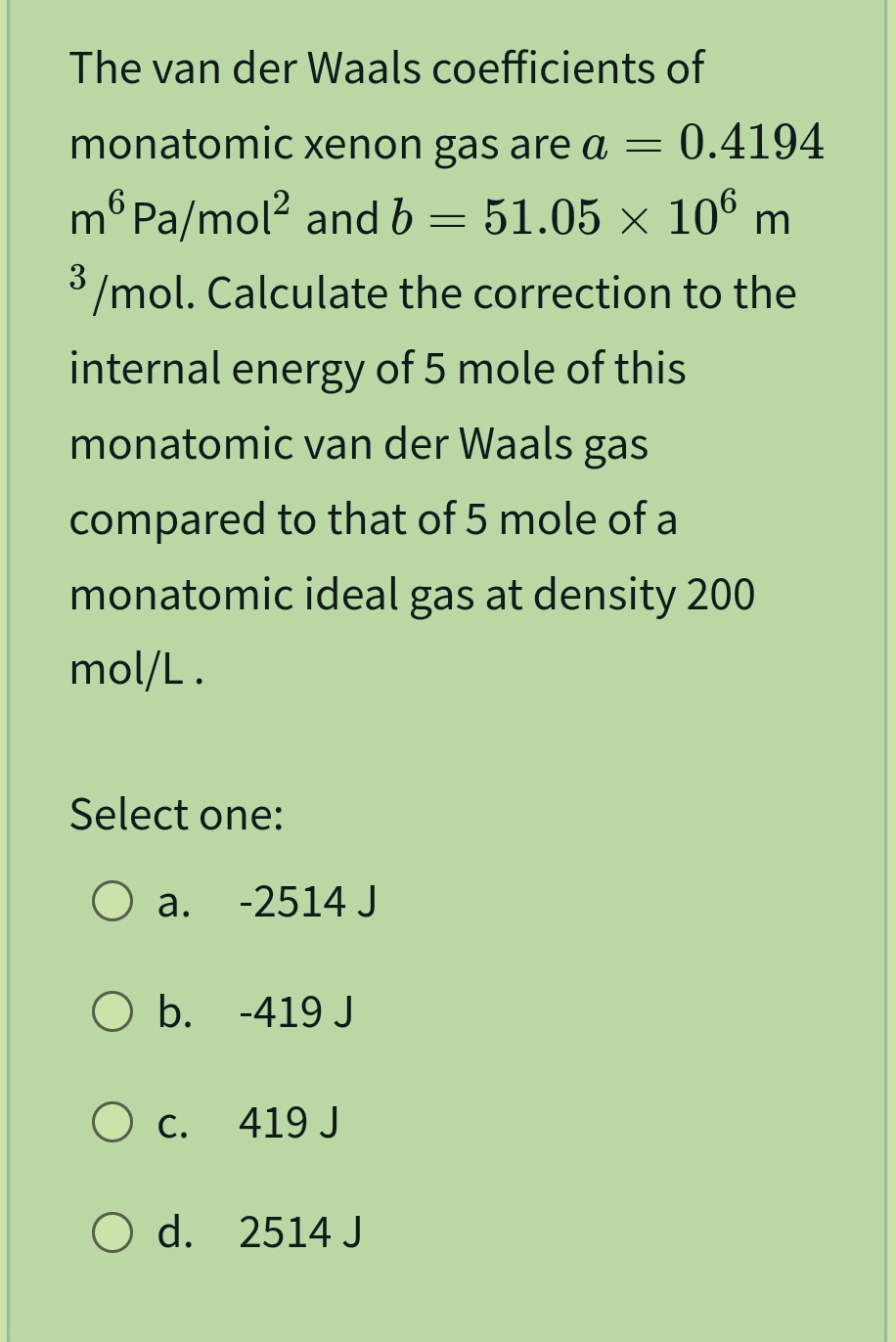
Answered: The van der Waals coefficients of…

Compressibility factor (gases) - Citizendium
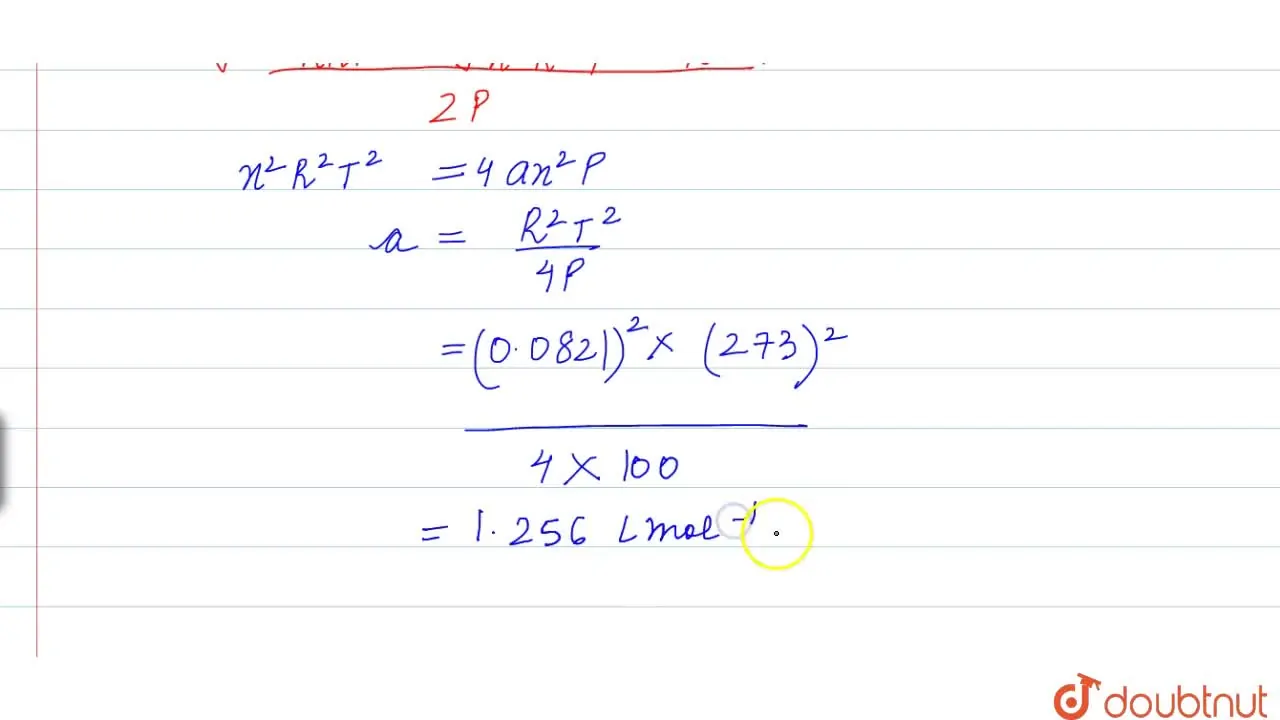
The compressibility factor for definite amount of van der Waals' gas a

2 mol of ammonia occupied a volume of 5 L at 27∘ C. Calculate the pressure if the gas obeyed van der Waals equation. a = .4.17 atm L 2 mol 2
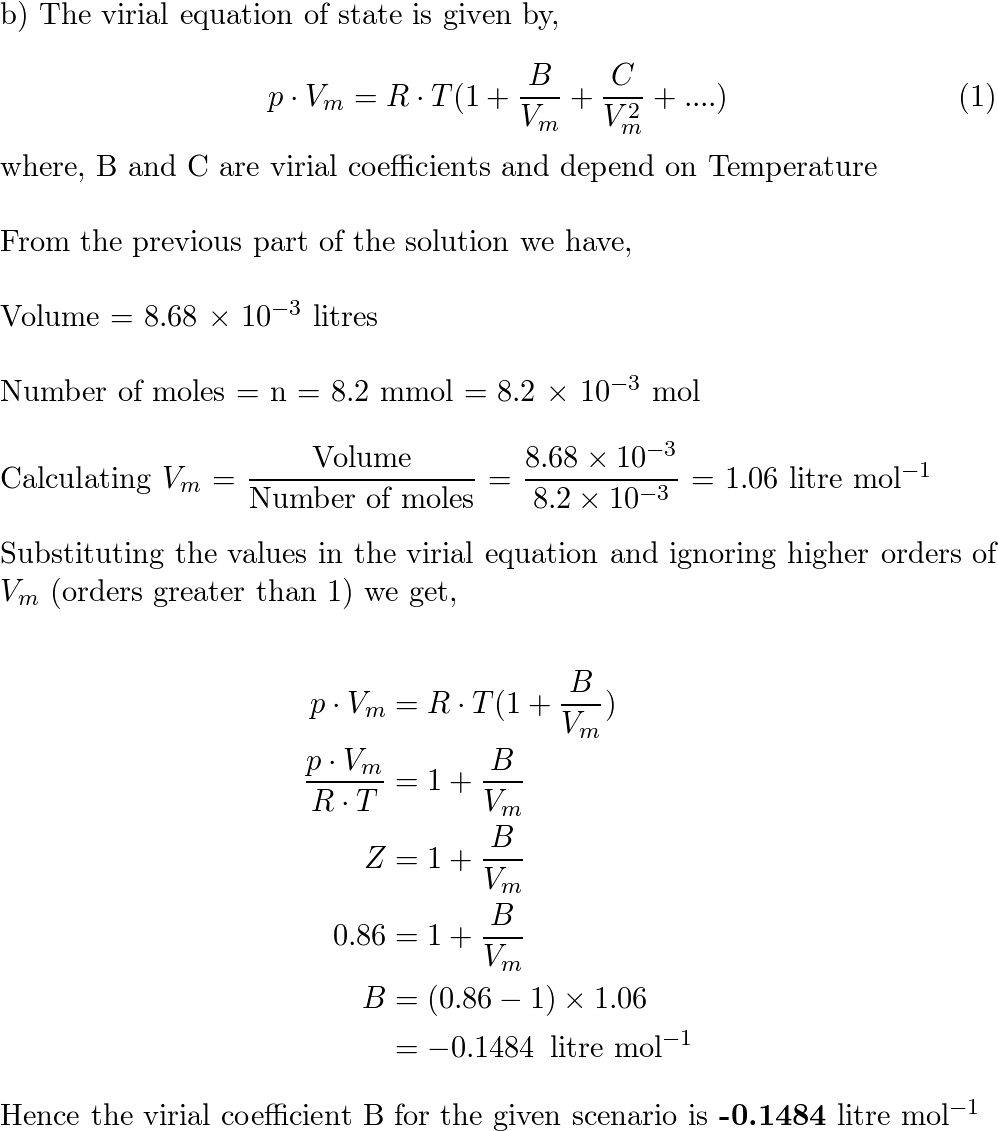

Density of van der Waals' gas 500 K and 1.0 atm was found to be 0.8 kg/m'. Also gas was found to effuse 1.37 times slower than oxygen under identical condition. Determine

Answered: Compression factor of a gas with van…

Physical Chemistry The Compression Factor (Z) [w/1 example]
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas

1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts

Poulduly 59. What is the compressibility fac is the compressibility factor (Z) 0.02 mole co Vanderwaals' gas pressure of 0.1 atm. Assume the size of gas molecules is negligible. . RT =
How to calculate the values of critical pressure and temperature for a given gas (Van der Waals equation) - Quora

The compression factor (compressibility factor) for one mole of a van der..
- Solved 2. By definition, the compression factor of an ideal

- What is the compression ratio, and how is it calculated? - Quora
- a) Suppose that $10.0\ \mathrm{mol}\ \mathrm{C}_{2} \mathrm
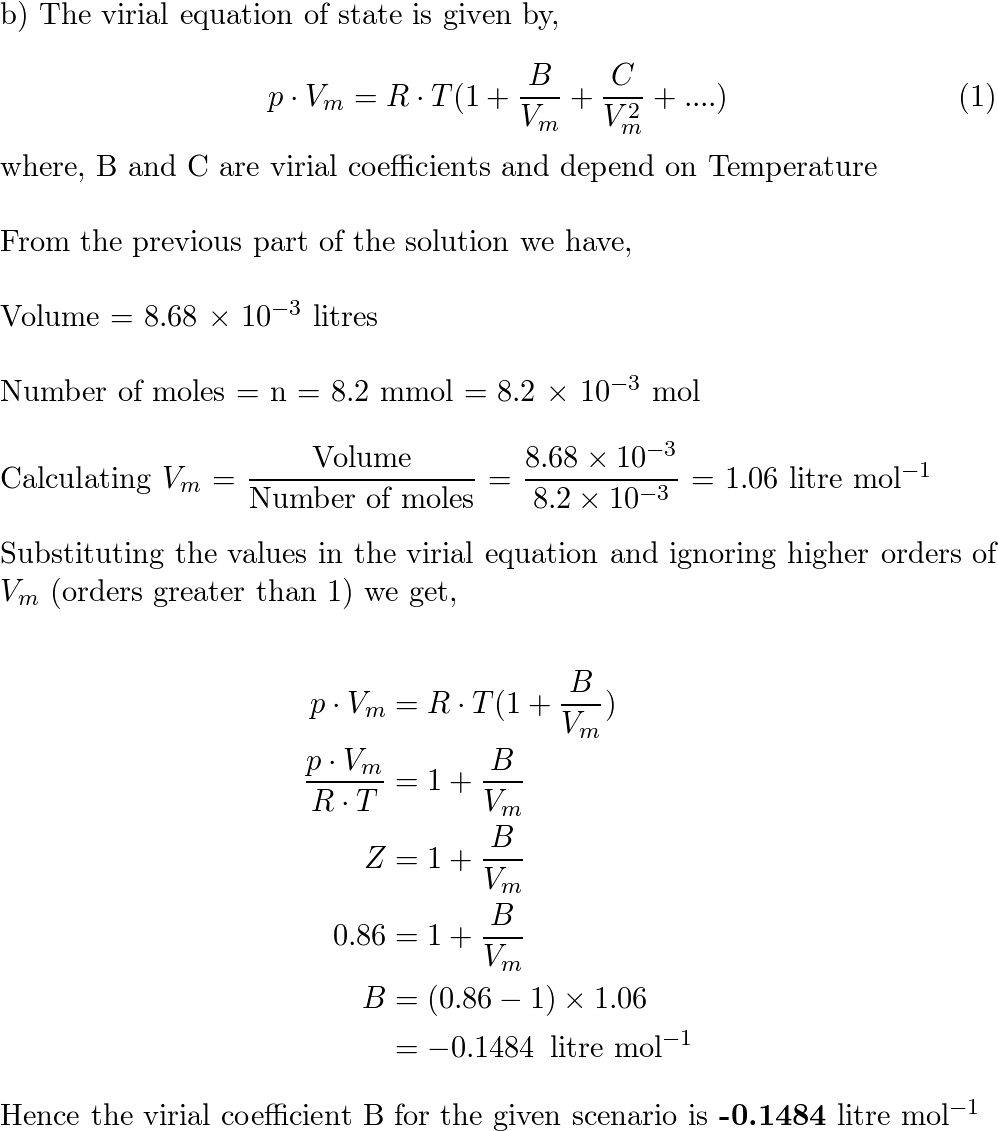
- SOLVED: For a gas at a given temperature, the compression factor is described by the empirical equation: z = 1 - 8.50 × 10^(-3)P/P° + 3.50 × 10^(-5)(P/P°)^2 where P° = 1

- Solved Define the compression factor, Z and explain its





