The correct order of increasing bond length of \( \mathrm{C
By A Mystery Man Writer
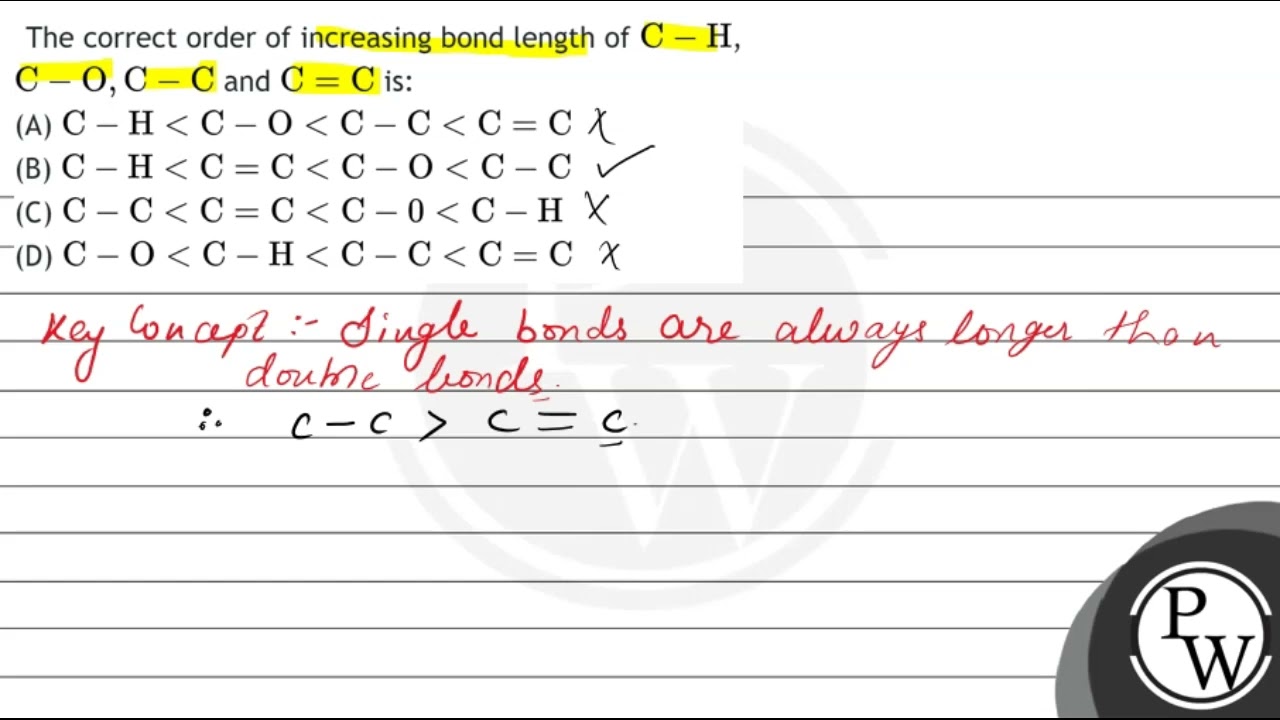
The correct order of increasing bond length of \( \mathrm{C}-\mathrm{H} \), \( \mathrm{C}-\mathrm{O}, \mathrm{C}-\mathrm{C} \) and \( \mathrm{C}=\mathrm{C} \
The stability of covalent dative bond significantly increases with increasing solvent polarity

The correct order of increasing bond length of C - H ,C - O, C - C and C = C is

Rank the following bonds in order of increasing bond length.

The decreasing order of bond length of C = C bond in the following compounds is
Which of the following has the longest bond length, C=O, N=O, C=C, or C=N? - Quora

⏩SOLVED:Using the periodic table only, arrange the members of each…

Which of the following has the longest bond length, C=O, N=O, C=C, or C=N? - Quora

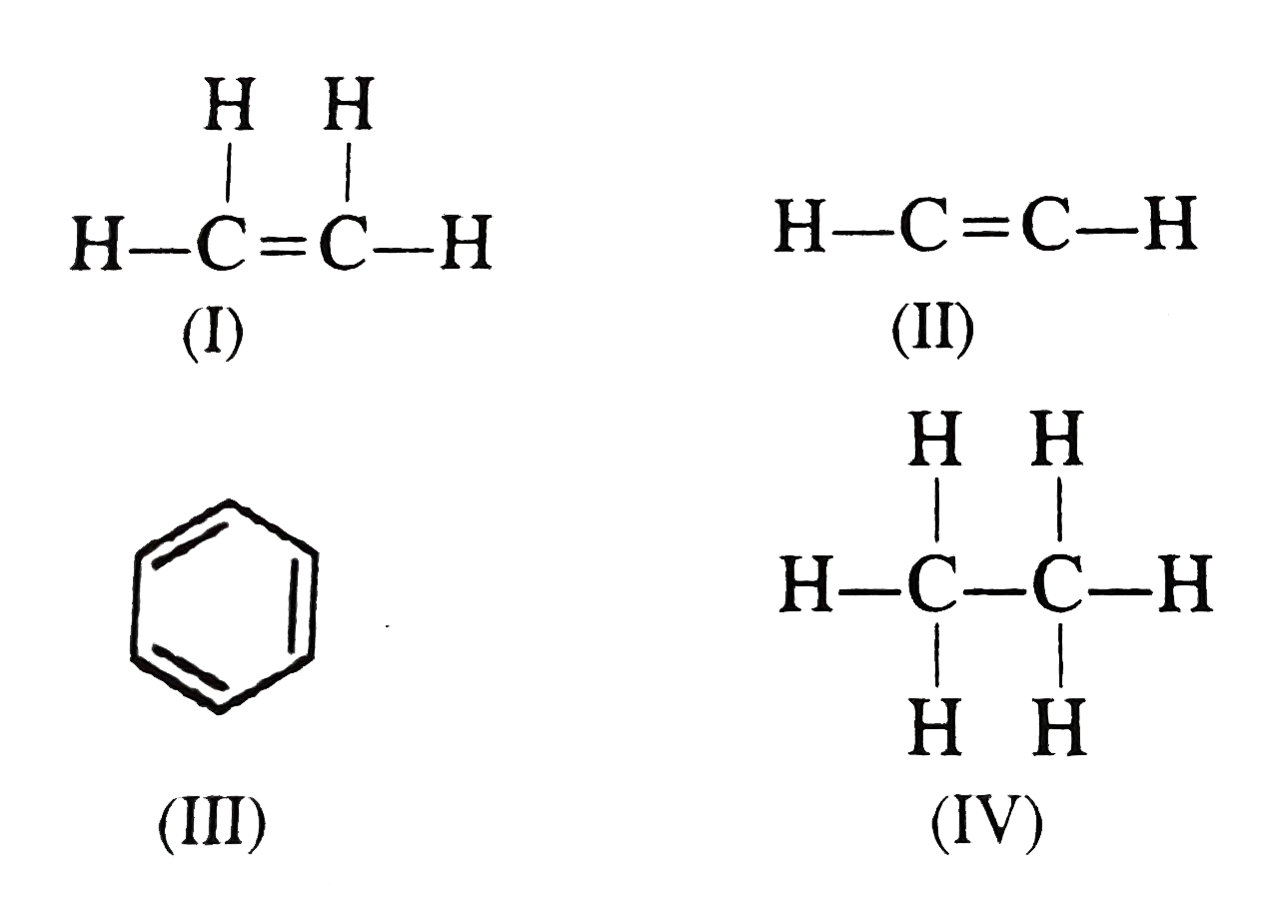
Decreasing order of C-C bond length is (I) C2H4 (II) C2H2 (III) C6 H6
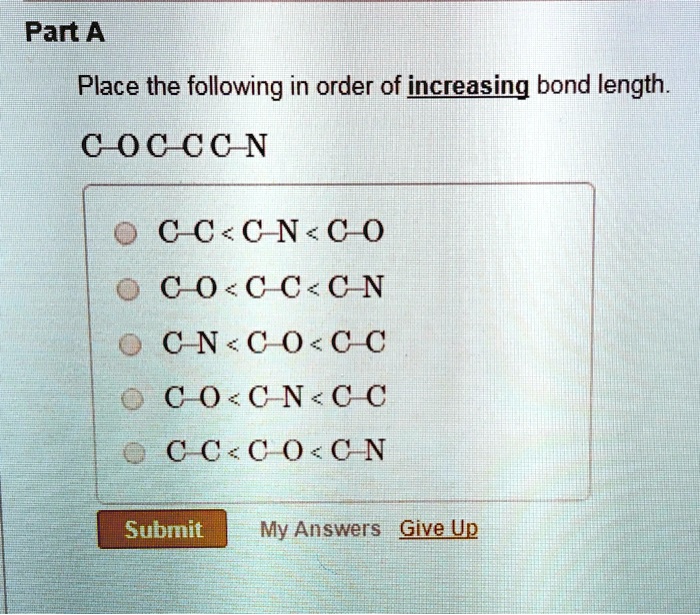
SOLVED: Part A: Place the following in order of increasing bond length. C-C < C=C < C-N < C=O
Protein primary structure - Wikipedia
- Solved FeBr3⟶Br2 F. F. G. 4. Which of the following is an
- CCC 144 TC Cotton Double Printed Flat Bedsheet - Buy CCC 144 TC Cotton Double Printed Flat Bedsheet Online at Best Price in India

- Erica Ellis, Ph.D., CCC-SLP - Associate Professor - California State University, Los Angeles
- A) Pseudocomplementary pairing of nA and sT. (B) A double D-loop

- 700W Thermal & Cold Laminator, Professional Film Laminating Machine, Roll Mount Laminator with Single/Double Side Heating, CE/FCC/CCC/PSE (30cm Width Laminator) : : Office Products