Friday, Sept 20 2024
Color change is only device modification. Is a new 510k required? - Medical Device Academy

By A Mystery Man Writer
This article explains the process for determining if a color change and other material changes require a new 510k prior to implementing the change.

FDA

Predicate selection guidance proposes controversial additions

New Guidance from FDA: When to Submit a 510(k) for a Change to a
FDA

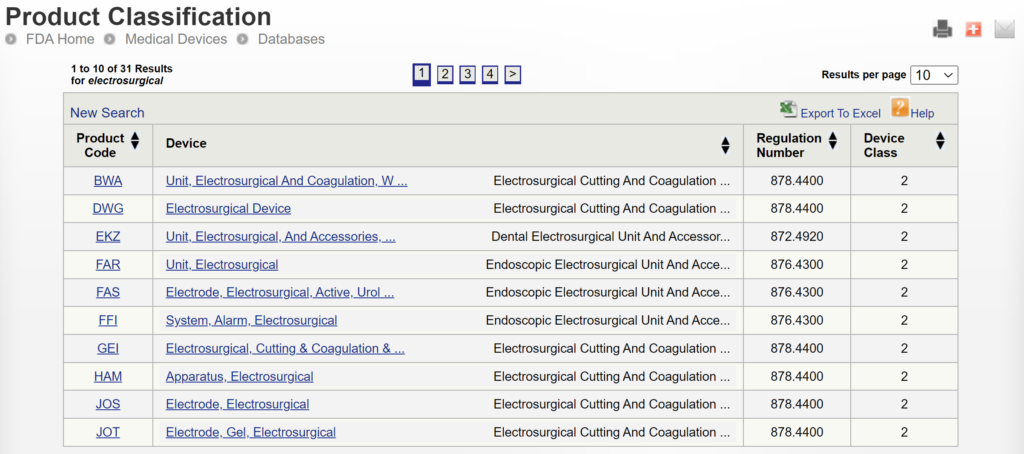
Medical device regulations, classification & submissions
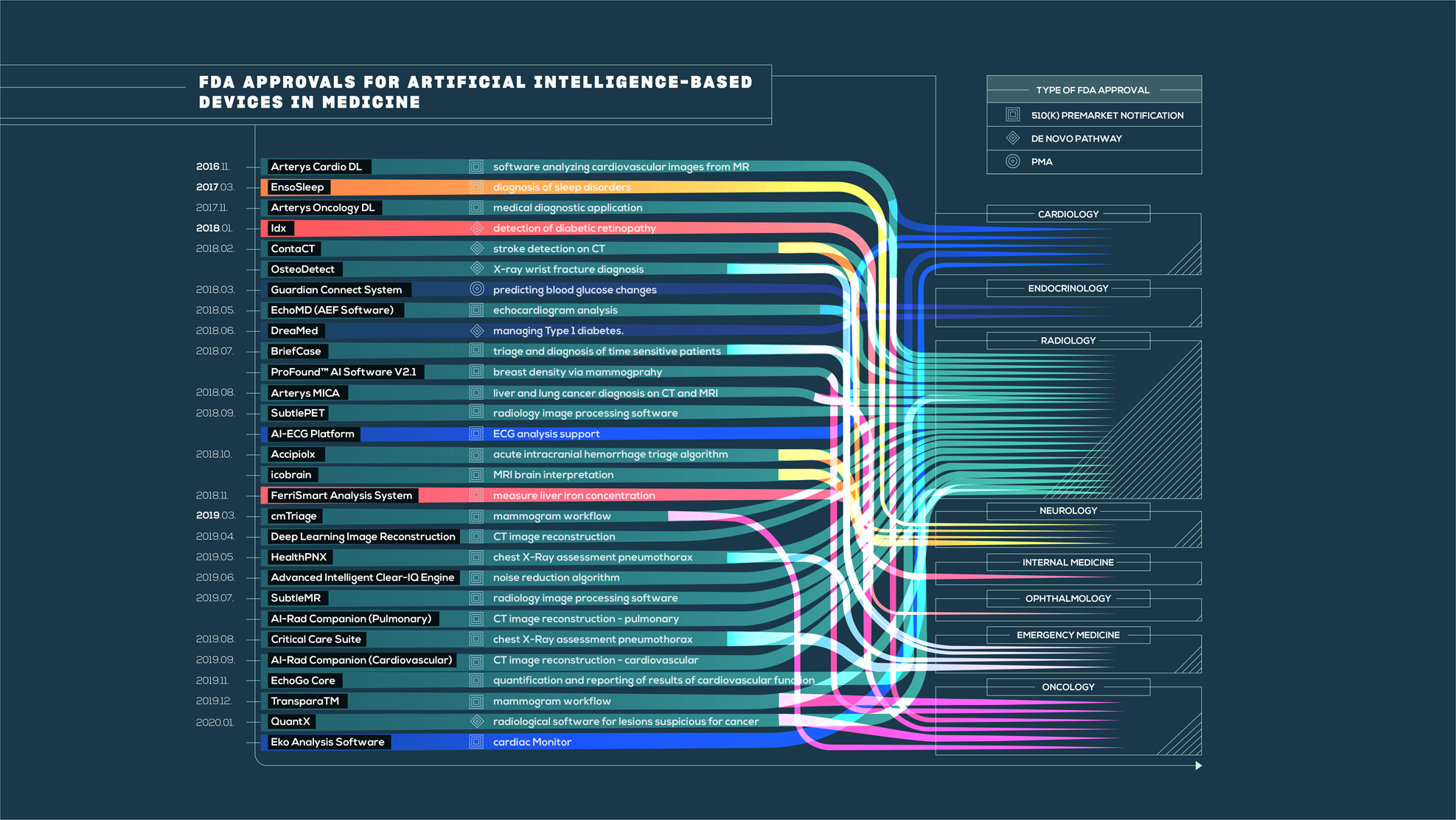
The state of artificial intelligence-based FDA-approved medical

What Should be Included in a 510k

4 Steps to Master Substantial Equivalence (510k process)

Your Medical Devices Are Getting Smarter. Can the FDA Keep Them Safe? - WSJ

UDI Procedure (SYS-39) and Webinar Bundle

Does Your Device Modification Qualify For A Special 510(k)?
Related searches
- Color Changing Self-Adhesive Vinyl - Vinyl Me Now

- Color Change Sapphire - Color First

- The Dress Resurrected: Color-Changing Shoe Breaks Internet — RADII

- NEON Changing Color - Flashing FLUO Lights - Colorful Lights - Fast colour changing screen - 80'

- How to make Color Changing Alcohol (Vodka, Tequila and Gin) - The Flavor Bender

Related searches
- Joy Lab Sports Bra Womens XL Navy Blue Sparkle V Neck Strappy Crossback

- Using the Ruby Ribbon Shape and Support Scale to Find What's Best

- Maidenform Womens Flexees Embellished Firm Control Bodysuit Style

- Sergio Hudson x Target SIZE 4X Women's Balloon Sleeve Midi Dress Pink Plus Size for sale online

- Plush Robe - Pillow Talk

©2016-2024, slotxogame24hr.com, Inc. or its affiliates