PDF] Hemoglobin polymorphism in white-tailed deer: subunit basis

By A Mystery Man Writer
It was concluded from the results of limited structural studies that there were multiple peptide differences upon comparison of three non-α polypeptide chains in white-tailed deer. A variety of aberrant erythrocyte forms have been related to seven adult and two fetal hemoglobins in white-tailed deer. While sickling of the erythrocyte was not associated with a single hemoglobin type, it was precluded by hemoglobin V or VII, even when in combination with other hemoglobin types normally associated with sickling. The subunit basis of the hemoglobin polymorphism was presented. Two kinds of α subunits, six kinds of β subunits and one γ subunit were related to the whole hemoglobin molecule. The heterogeneity of the deer hemoglobins was based upon a variety of combinations of these numerous polypeptide chains. It was concluded from the results of limited structural studies that there were multiple peptide differences upon comparison of three non-α polypeptide chains.

Relaxed functional constraints on triplicate α-globin gene in the

Diversity, Free Full-Text

Ultrastructure of Sickled Deer Erythrocytes. I. The Typical

High-altitude deer mouse hypoxia-inducible factor-2α shows

A Structural Analysis of the FDA Green Book-Approved Veterinary

Lubert Stryer - Biochemistry.pdf
Alteration of the α1β2/α2β1 subunit interface contributes to the

(PDF) Pan-American Trypanosoma (Megatrypanum) trinaperronei n
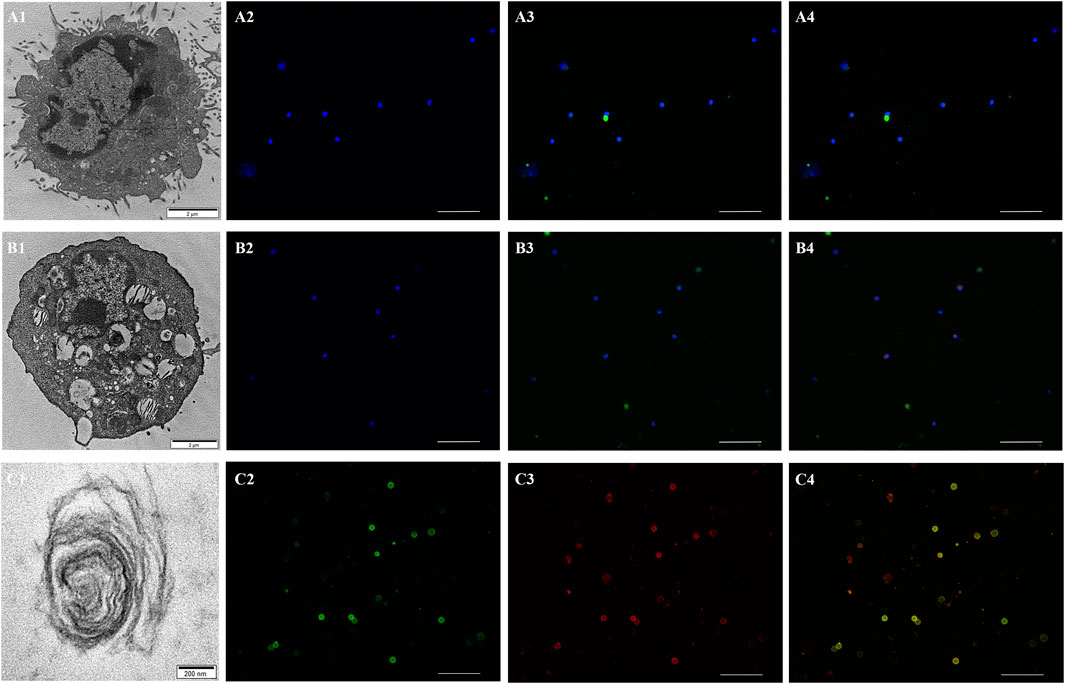
Frontiers A New Homotetramer Hemoglobin in the Pulmonary

Multiplicity and Polymorphism of Fish Hemoglobins
- HEMO Shapewear Women's Tummy Control Shapewear Long Pant Trainer

- Curved Rubber Hemo Split Bard Permanent Catheter, Size: Medium at

- HEMO Shapewear Women's Slimming Shapewear for Women Bummach

- HEMO Body Saper Bodysuit Body Shaper Hip Enhancer Weight Loss Shapewear Padded Panties Fake Ass Butt Lifter For Lady Plus Sizes Shape Wear (Color : Apricot, Size : XXL) : : Fashion

- Curved Rubber Hemo Split Bard Permanent Catheter, Size: Medium at Rs 11500 in Navi Mumbai





