What is the mass of glucose required to produce 44g of C{O_{2'}} on complete combustion?30g45g60g22g

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:what is the mass of glucose required to produce 44g of co2 on complete
Click here👆to get an answer to your question ✍️ What is the mass of glucose required to produce 44g of C-O-2- on complete combustion-30g45g60g22g
Solved Consider the combustion of 5.000 g of glucose

What is the mass of glucose required to produce 44 g of CO(2), on comp

SOLVED: For the combustion reaction: C6H12O6 + 6 O2 -> 6 H2O + 6 CO2 If you burn 100 g of glucose (C6H12O6), how many grams of CO2 are produced? Problem-solving pathway

Solved In the combustion analysis of 0.1127 g of glucose

Mass of glucose required to produce 44g of co2 on complete oxidation?
Wkst Stoich - CHM 130 Stoichiometry Worksheet The following flow chart may help you work - Studocu
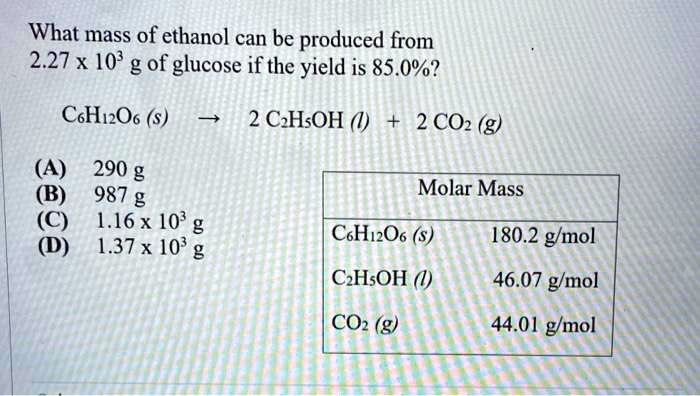
SOLVED: What mass of ethanol can be produced from 2.27 x 10^9 g of glucose if the yield is 85.0%? C6H12O6 â†' 2 C2H5OH + 2 CO2 (g) Molar Mass: C6H12O6 =
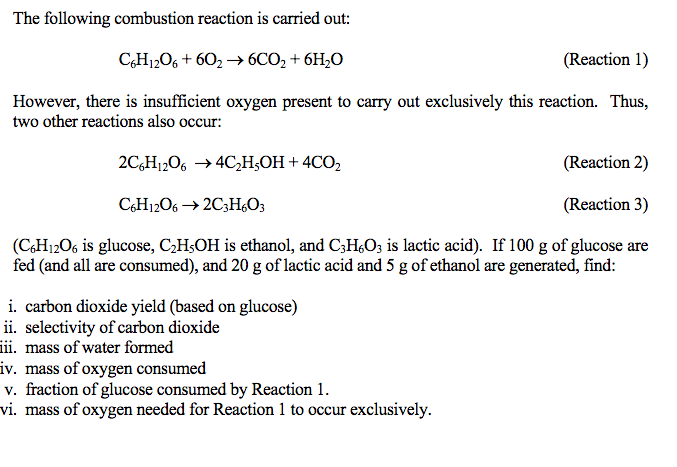
Solved The following combustion reaction is carried out
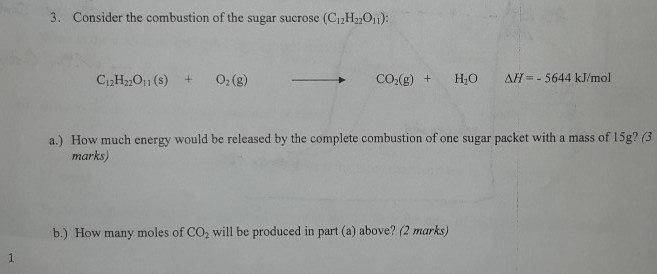
Solved 3. Consider the combustion of the sugar sucrose

Determine the mass of CO2 produced by burning enough of each fuel

Solved 1. The combustion of sugar produces carbon dioxide
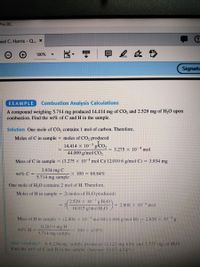
Answered: TEST YOURSELF A 6.234-mg sample…
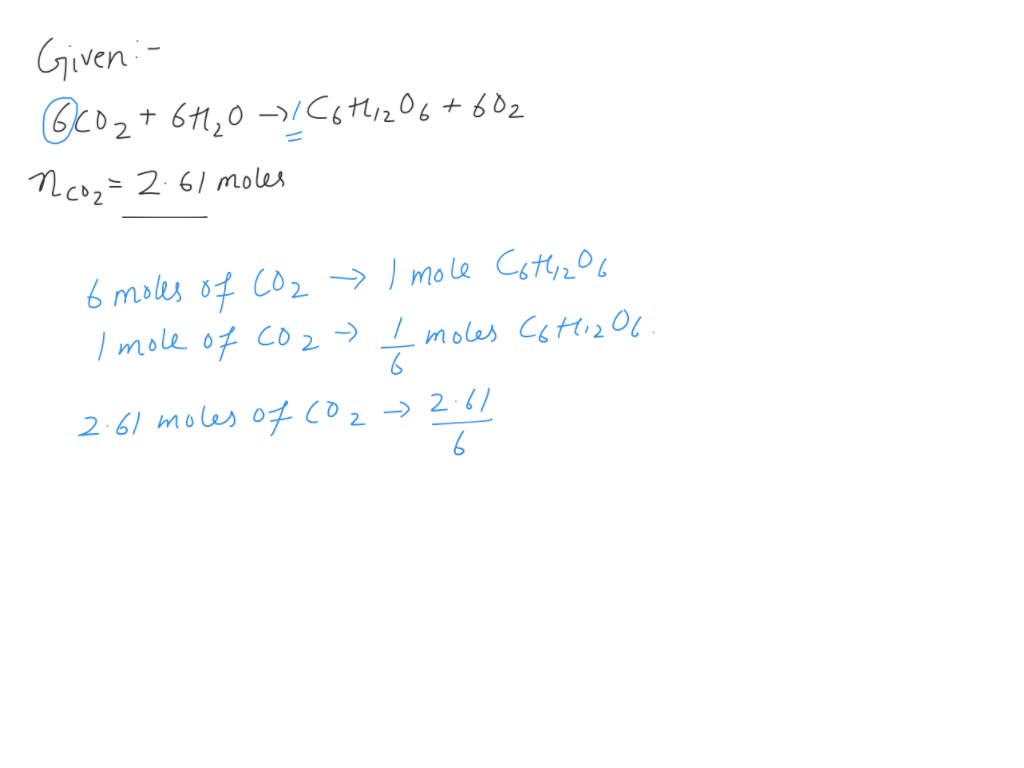
SOLVED: Question 18 (Mandatory) (2.5 points) Glucose (C6H12O6) is an important energy-rich compound produced by photosynthesis according to the equation below. What mass of glucose can be produced from 2.61 mol of
- I'm 5'7” with 44G boobs – I did a Target bikini haul, the leopard bottoms were 'so raunchy' I had to wear them backward

- What is the number of atoms in 100 grams of CO2? - Quora
- 2023 Toyota Tundra with 22x12 -44 G-FX Tm6 and 305/45R22 General Grabber Uhp and Leveling Kit

- Drogaria Santa Terezinha

- Hangar 44: Luxury Apartments for Rent in Phoenix, AZ





