Aβ(1-42) tetramer and octamer structures reveal edge conductivity pores as a mechanism for membrane damage
By A Mystery Man Writer

Effects of the Hydrophilic N-Terminal Region on Aβ-Mediated

Atomic Structure of Alzheimer's Amyloid Protein Reveals New

The amyloid concentric β-barrel hypothesis: Models of amyloid beta

IJMS, Free Full-Text
Aβ-Peptide Production and Conformational Behavior
Molecules, Free Full-Text

Analyzing Morphological Properties of Early-Stage Toxic Amyloid β

Journal of Cellular Biochemistry
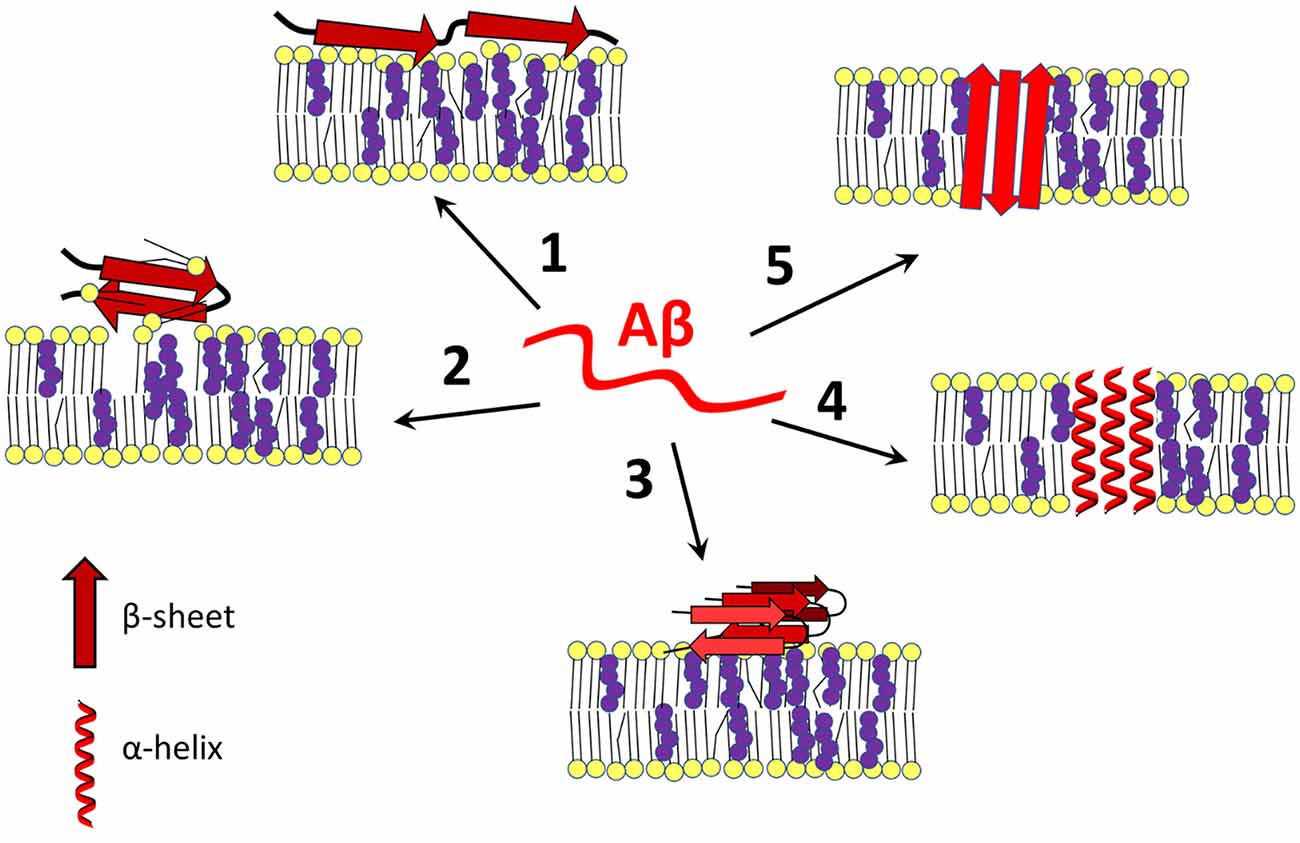
Frontiers Cholesterol as a key player in amyloid β-mediated

Aβ42 purification. (A) SDS-PAGE analysis of SUMO-Aβ42 fusion
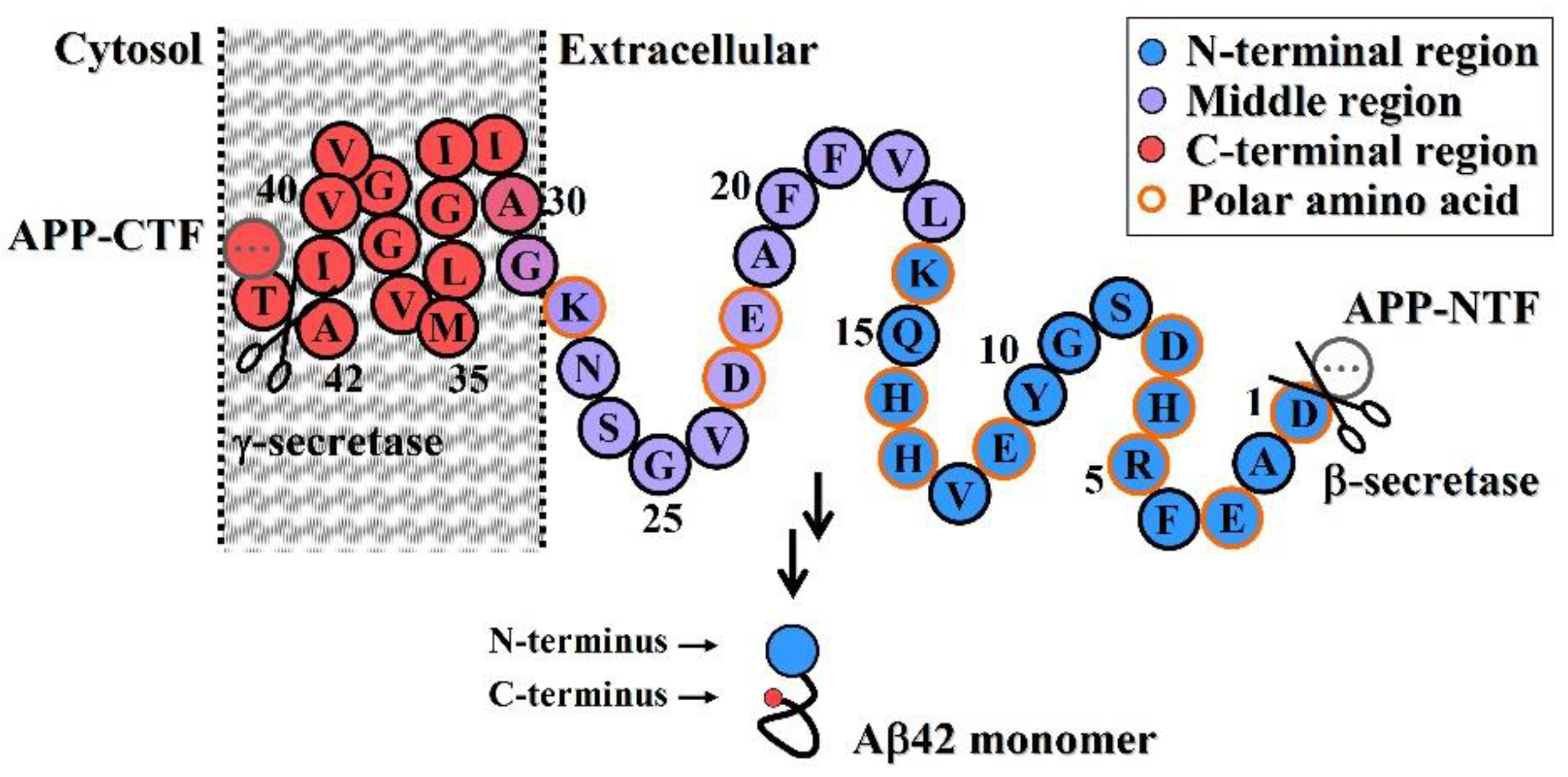
Molecules, Free Full-Text

RCSB PDB - 6RHY: Structure of pore-forming amyloid-beta tetramers
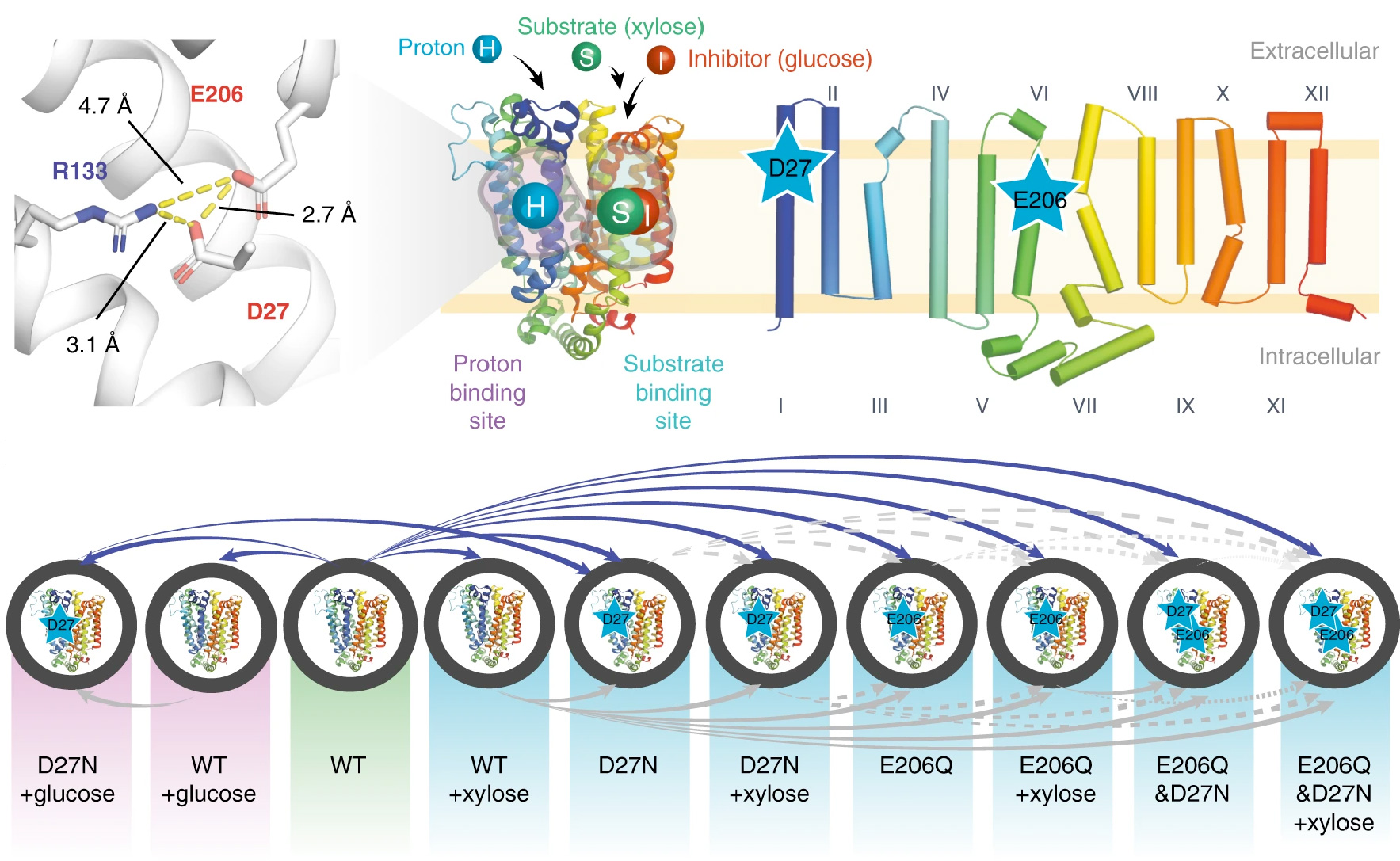
Computational Structural Biology and Molecular Biophysics

Aβ(1-42) tetramer and octamer structures reveal edge conductivity

In vivo induction of membrane damage by β-amyloid peptide