Friday, Sept 20 2024
physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

By A Mystery Man Writer
The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate. In

3.2 Real gas and compressibility factor – Introduction to

Compressibility Factor - an overview
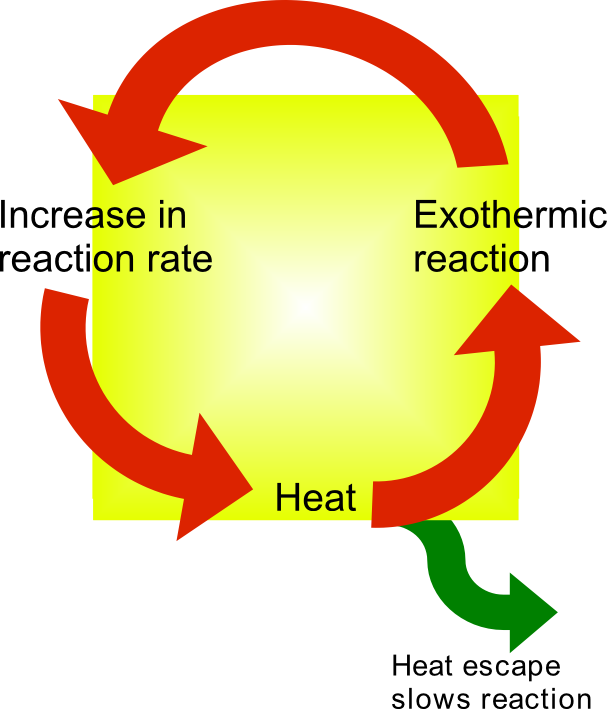
Thermal runaway - Wikipedia

gas laws - Which gas is easier to compress, the ideal gas or a
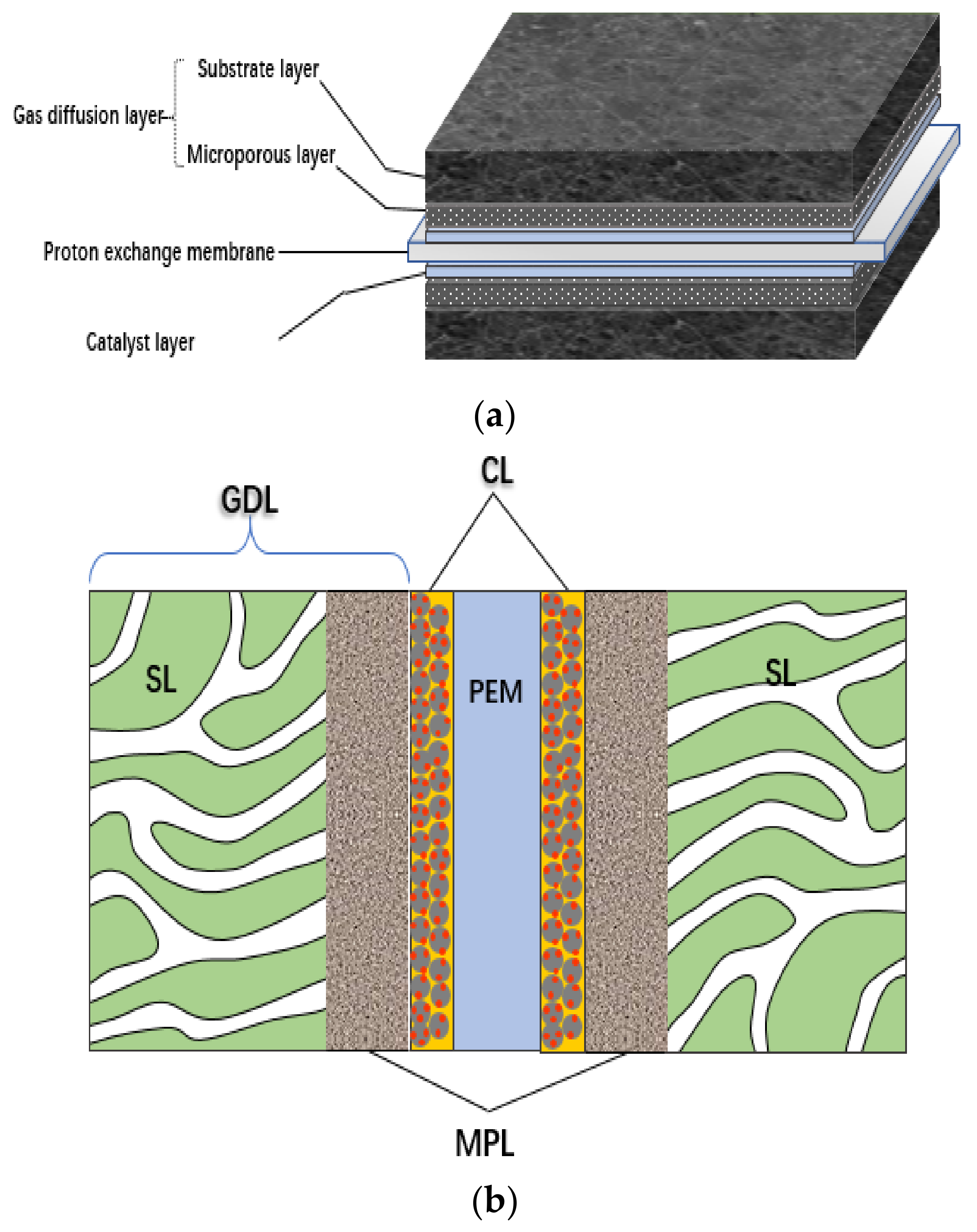
Membranes, Free Full-Text
Electrochemical Compression Technologies for High-Pressure
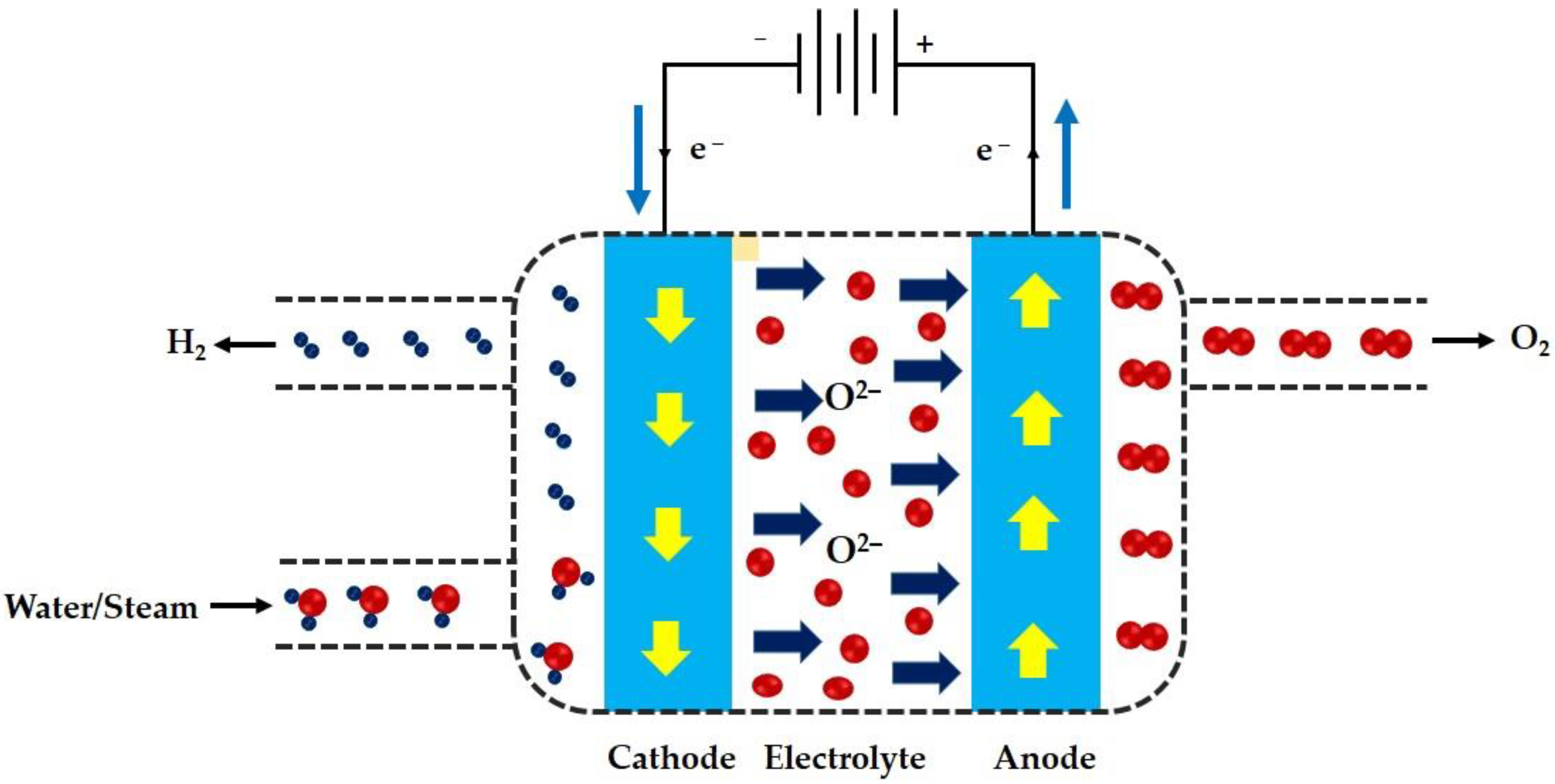
Energies, Free Full-Text
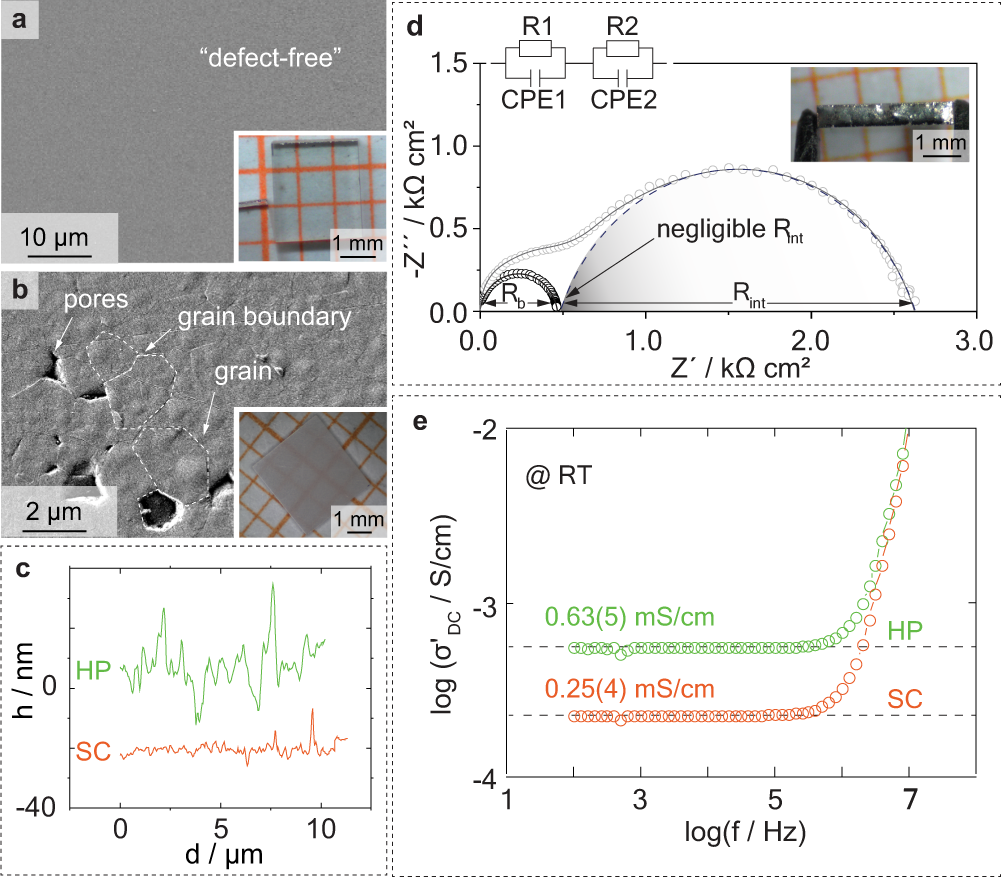
Effect of pulse-current-based protocols on the lithium dendrite

Fuel cell - Wikipedia

physical chemistry - Pressure vs volume plot for real gas and
Related searches
- Solved Using the chart, the compressibility factor (Z), for
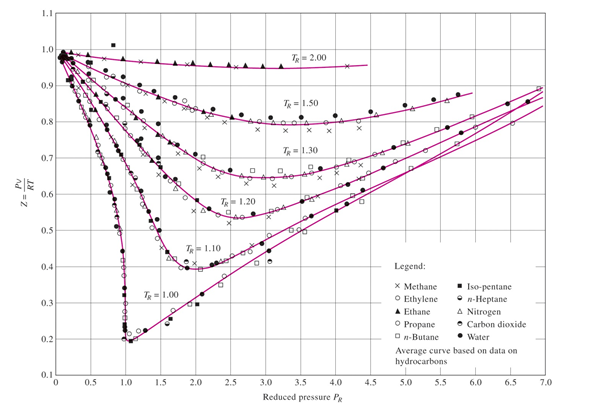
- e Compressibility factor (Z) for hydrogen WRT pressure and temperature

- My publications - CHM 201-LECTURE IV-REAL GASES - Page 8 - Created with Publitas.com

- Two extensions of the compressibility factor Z correlation (sub
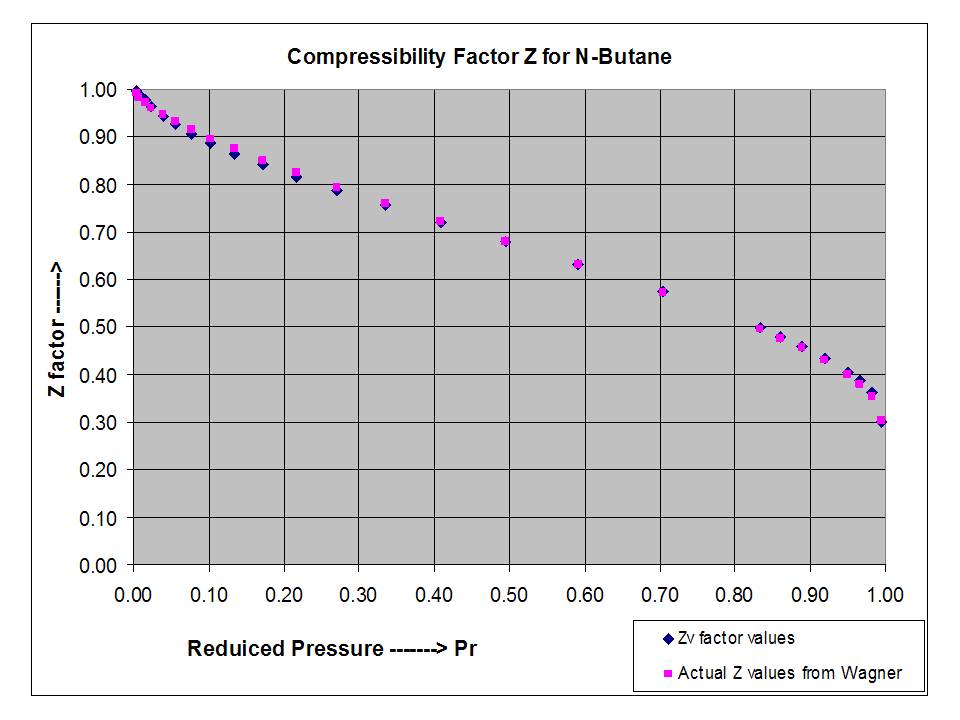
- In the above figure, near the point B, compressibility factor Z is about..
©2016-2024, slotxogame24hr.com, Inc. or its affiliates




