SOLVED: For a gas at a given temperature, the compression factor is described by the empirical equation: z = 1 - 8.50 × 10^(-3)P/P° + 3.50 × 10^(-5)(P/P°)^2 where P° = 1

By A Mystery Man Writer
VIDEO ANSWER: Hello students: let's look at the question: l n, that integrate integration and 0 z minus 1 bracket, close d p by p here. Minus 1 is equal to minus 8.50 into 10 to the power minus 3 p by p, not plus 3.50 into 10. To the power minus 9. P
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.

Thermodynamics of Physical and Chemical Transformations
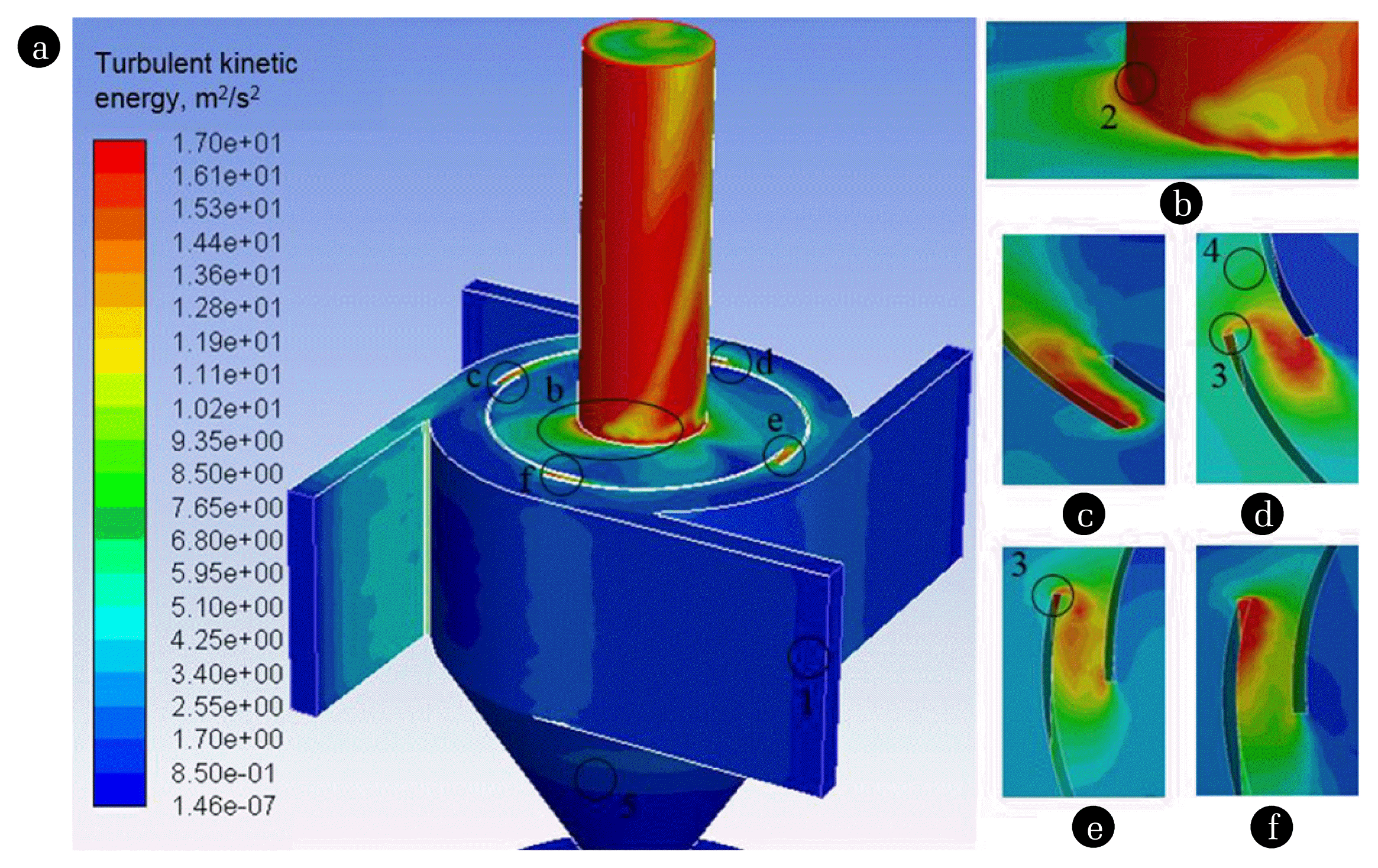
Investigation on particulate matter and gas motion processes in the advanced multi-channel cyclone-separator with secondary gas inlets
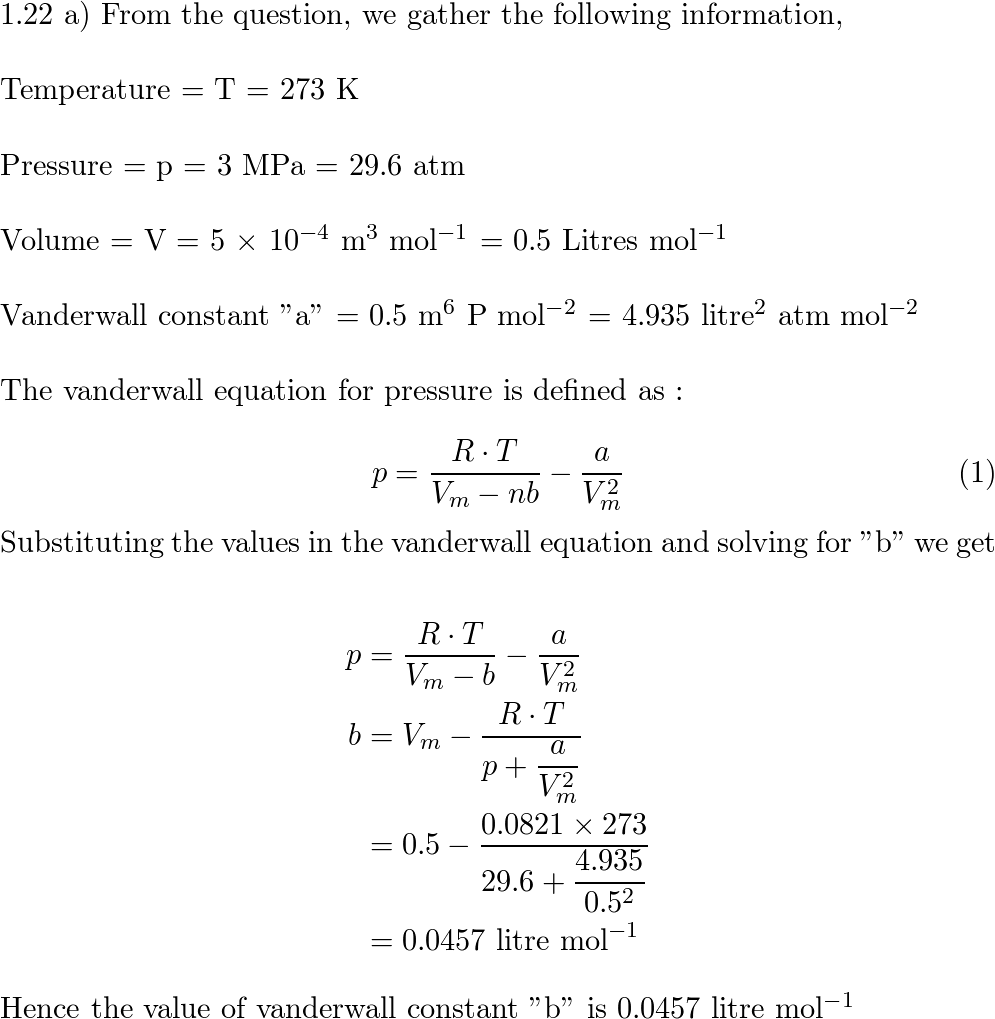
a) A certain gas obeys the van der Waals equation with $a =

300 Solved Problems in Geotechnical Engineering

Computation of The Compression Factor An, PDF, Gases
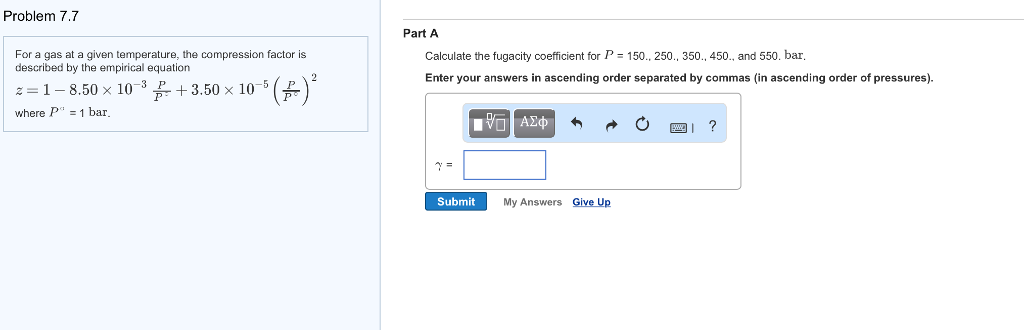
Solved Problem 7.7 Part A Calculate the fugacity coefficient

Pchem Instructor Solutions, PDF, Gases
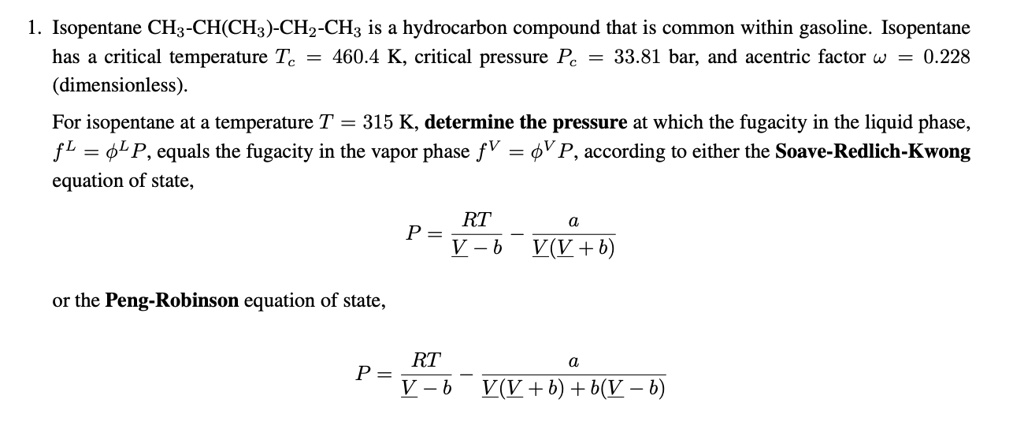
SOLVED: Isopentane (CH3-CH(CH-CH-CH3) is a hydrocarbon compound that is common within gasoline. Isopentane has a critical temperature (Tc) = 460.4 K, critical pressure (Pc) = 33.81 bar, and acentric factor (ω) =

Ficoquimica, PDF, Gases

10. Appendix

Multiparticle Effective Field and Related Methods in Micromechanics of Random Structure Composites

Swirling and cavitating flow suppression in a cryogenic liquid turbine expander through geometric optimization - Peng Song, Jinju Sun, Ke Wang, 2015
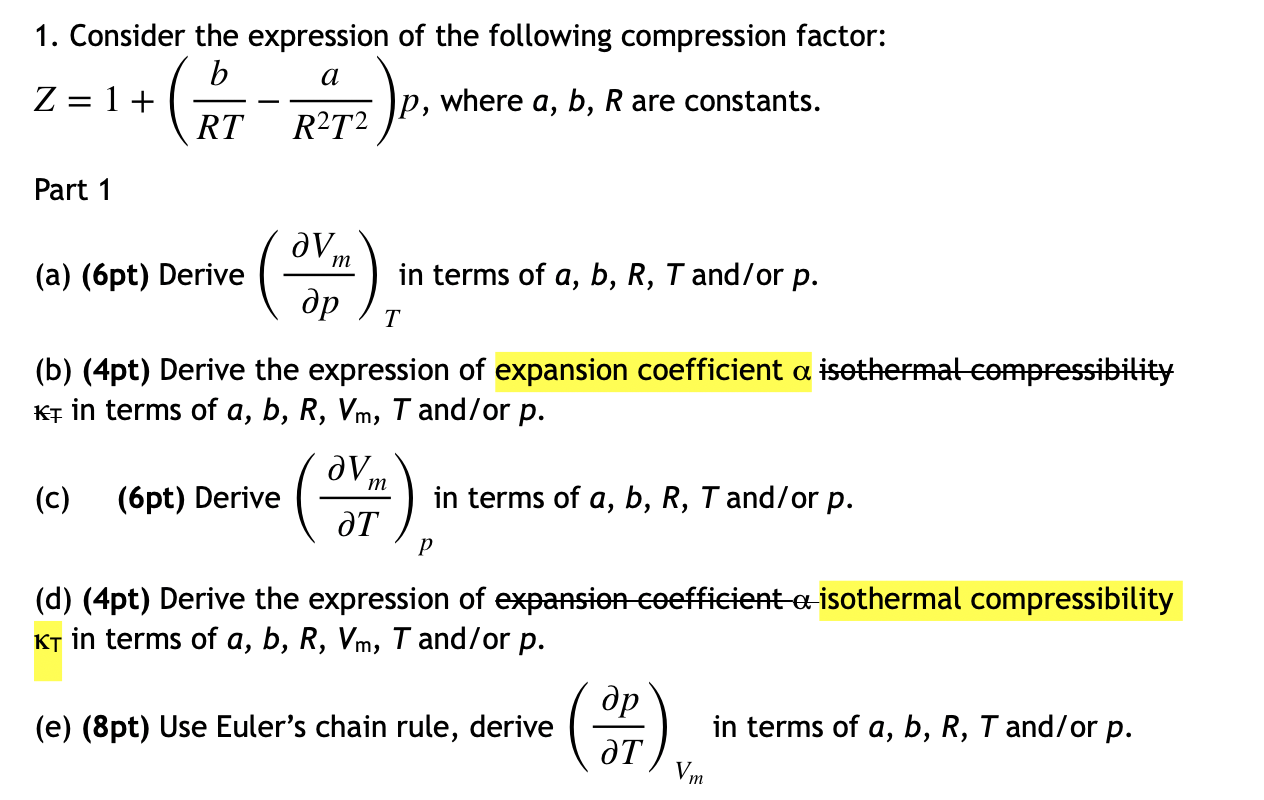
- Solved 1. Consider the expression of the following
- Telugu] What is compressiblity factor?

- What is the value of compression factor Z for the gas? (A) 1 (B) >1 (C) <1 (D) Zero

- Compared with the graph of the parent function, which equation shows only a vertical compression by a

- 53 pts!! The function f(x)= 7^x+1 is transformed to function g through a horizontal compression by a factor