For $CO$, isotherm is of the type as shown. Near the point compressibility factor $Z$ is?\n \n \n \n \n 1.$\\left( {1 + \\dfrac{b}{V}} \\right)$ 2.$\\left( {1 - \\dfrac{b}{V}} \\right)$3.$\\left( {1 + \\

By A Mystery Man Writer
For $CO$, isotherm is of the type as shown. Near the point compressibility factor $Z$ is?\n \n \n \n \n 1.$\\left( {1 + \\dfrac{b}{V}} \\right)$ 2.$\\left( {1 - \\dfrac{b}{V}} \\right)$3.$\\left( {1 + \\dfrac{a}{{RTV}}} \\right)$4.$\\lef

Solved The plot below shows how compressibility factor (Z)

Thermodynamics - Test 1 Problem 5 - Ideal Gas Equation with Compressibility Factor

EGR 334 Thermodynamics Chapter 3: Section ppt video online download

Gas compressibility factor Z: Ideal gas vs Real gas

Compressibility factor - Wikipedia

Isothermal Compressibility. - an overview

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Compressibility factor - Wikipedia
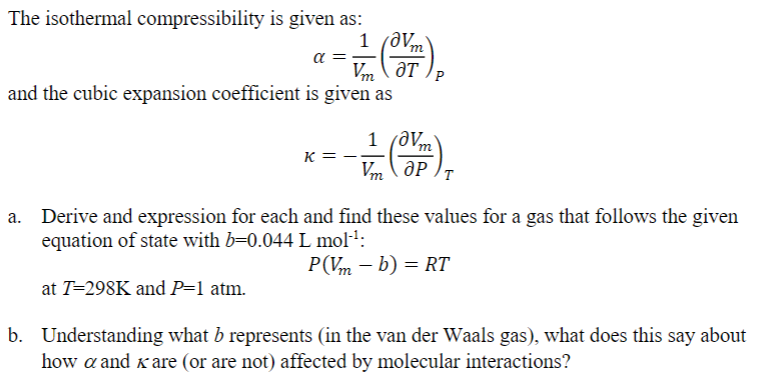
Solved The isothermal compressibility is given as

Compressibility factor - Wikipedia
For CO, isotherm is of the type as shown: Near the point A, compr
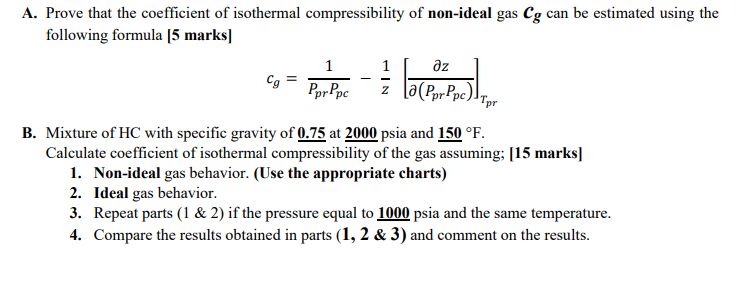
A. Prove that the coefficient of isothermal
- Determine Compressibility of Gases

- Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

- For $CO$, isotherm is of the type as shown. Near the point

- Compressibility factor Z for CO2

- PDF] Two Simple yet Accurate Equations for Calculating the Fugacity Coefficient Phi and the Gas Compressibility Factor

- VerPetridure Nursing Bras for Breastfeeding Plus Size High Support Comfort Maternity Bra,Seamless Soft Wirefree Pregnancy Bra

- Wacoal How Perfect Non-Wire Bra 852189

- Lululemon On the Fly Wide-Leg 7/8 Pant, The 205 Best Cyber Monday Deals People Are Already Shopping

- Knitted V Neck Sweater and Pants Lounge Set
- Rihanna's Inclusive Lingerie Line Is Making Some People Emotional
